1. Introduction
Polyphenols are a diverse group of plant secondary metabolites, which primary function is to protect plants against harmful ultraviolet UV radiation and pathogen attack [
1]. In the plant kingdom, these biomolecules also play the role of factors that determine their color and taste as well as increase their attractiveness for pollinating insects [
1]. Due to the ubiquitous presence of polyphenols in the plant world, they are an integral part of foods. Although these compounds do not exhibit nutritional functions in the body, numerous literature reports present them as valuable ingredients of the diet with health-promoting activities. Therefore, they are classified as bioactive phytochemicals that support the physiological functions of the body [
2]. Polyphenols are also generally considered to be non-toxic to the human body. Because their lethal doses are very high, their oral overdose is extremely difficult to achieve [
3].
Many literature data show that the estimated daily intake of polyphenols contained in fruits, vegetables, and beverages may reach approximately the level of 1700 mg [
4]. This total dietary polyphenolic fraction consists of compounds belonging to flavonoids, stilbenes, lignans and phenolic acids [
5]. The concentration of individual classes varies according to the source; however, it is worth noting that almost half of the daily dietary intake dose of polyphenols are phenolic acids (800 mg), mainly cinnamic acid derivatives (700 mg) and to a lesser degree, benzoic acid derivatives (100 mg) [
4]. Therefore, the high content of cinnamates in natural products and in our diet indicates their significant contribution to the health-promoting potential of plants.
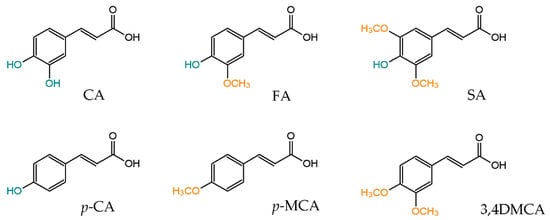
Phenylpropenoic acids commonly found in foods in the terms of chemical structure are hydroxy and methoxy derivatives of cinnamic acid. The most widespread like caffeic acid (CA), ferulic acid (FA), synapic acid (SA),
p-coumaric acid (
p-CA),
p-methoxycinnamic acid (
p-MCA) and 3,4-dimethoxycinnamic acid (3,4DMCA) are shown in . These compounds are present in many food products, but berries, cereal, beverages and spices are considered as their richest sources [
6,
7,
8]. The first one, caffeic acid (CA), is mainly present in coffee [
9] and regular drinking of this beverage can supply from 250 to 500 mg of CA per day [
10]. Coffee is also a valuable source of methoxy derivatives of cinnamic acid such as
p-MCA and 3,4DMCA (690 mg/kg dry weight of coffee beans) [
9]. Synapic acid (SA) and
p-coumaric acid (
p-CA) are compounds in which significant amounts have been determined in fruits [
11]. Black mulberry fruits (1448 mg/kg dry weight) and strawberries (1107 mg/kg dry weight) are particularly rich sources of
p-CA, while synapic acid has been found mainly in apples and pears, and its content can range from 15 to 600 mg/kg dry weight depends on their species [
11]. Ferulic acid is commonly found in cereal raw materials such as corn, barley, wheat, rice and whole-grain bread where its content reaches up to 330 mg/kg of dry weight [
12].
Figure 1. Chemical structure of dietary phenylpropenoic acids.
In most natural sources phenylpropenoic acids are rarely found in free form. Russell et al. reported that the concentration of their bounded form is even several hundred times higher than the concentration of their free forms [
6]. This is the result of the role that these compounds play in plants, defend them against pathogen attack. A significant accumulation of phenylpropenoic acids is observed in the area of the plant cell walls. They occur there as the complexes with other compounds forming the scaffold of biological structures which are resistant to chemical and physical factors. This significantly limits the bioavailability of these acids by humans because in this form they are not directly absorbed from the gastrointestinal tract. Thus, their absorption is possible only after release by hydrolysis processes catalyzed mainly by intestinal microflora enzymes [
6].
Many of the phenylpropenoic acids including (especially) the methoxy derivatives of cinnamic acid—ferulic acid,
p-methoxycinnamic acid and 3,4-dimethoxycinnamic acid—are also active compounds of medicinal plants such as: angelica sinensis (
Angelica sinensis), common columbine (
Aquilegia vulgaris), hogweed (
Cimicifuga heracleifolia), leprechaun (
Scrophularia buergeriana) or kencur (
Kaempferia galanga). These plants have been used for centuries in Eastern medicine as natural remedies for preventing and treating many diseases related to the function of the digestive, respiratory, nervous and immune systems [
3,
11,
12,
13,
14,
15,
16].
2. Biological Activity of Methoxylated Derivatives of Cinnamic Acid
The biological activity of compounds highly depends on their chemical structure, which affects such parameters as solubility, ability to penetrate biological membranes or binding to the active center of enzymes. In the case of natural derivatives of cinnamic acid, their bioactivity is mainly determined by the presence of hydroxy (-OH) and methoxy substituents (-OCH3) in the aromatic ring, which number and site of substitution define the wide range and potency of their action.
One of the first reports in the literature on the relationship between structure and biological potential of methoxy derivatives of phenylpropenoic acids dates back to the 1970s, when Japanese scientists published a report on the strong antioxidant activity of ferulic acid [
17]. They proved that this phenolic acid exhibits chain-breaking activity and prevents ischaemia–reperfusion-associated intestinal injury [
18]. A few years later, ferulic acid was accepted as an antioxidant food additive in Japan [
19]. In the following years, it also became the subject of worldwide experiments, which confirmed its antimicrobial [
20] and anticancer (against lung [
21], breast [
22], cervical [
23], prostate [
24], thyroid [
25] and gastrointestinal [
26] cancers) properties in in vitro tests. Furthermore, such beneficial activities as antidiabetic [
27] hepato- [
28], cardio- [
29] and neuroprotective [
30] activities were also confirmed during the in vivo tests in animal models. Of particular interest seems to be the anti-atherosclerotic activity of ferulic acid, which during studies in mice, showed a more potent effect than reference compound—clofibrate, a drug commonly used to reduce blood cholesterol level. It was reported that FA significantly increased the activity of hepatic and erythrocyte antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and paraoxonase) that inhibit the oxidation of low-density lipoprotein (LDL), and thus showed potent anti-atherosclerotic activity [
29]. Currently, a ferulic acid-based preparation is available in China and has found application in the treatment of cardiovascular diseases. However, due to the low bioavailability of its active ingredients, very high doses of this preparation are necessary to be administrated to achieve a therapeutic effect [
31].
Comprehensive studies on the relationship between the presence of a methoxy group in the aromatic ring of phenylpropenoic acids and their biological activity have been conducted by the Adisakwattana team between 2004 and 2017. As a result of a series of in vitro and in vivo tests, the researchers observed that the presence of the -OCH
3 group in the
para position is a key structural element that determines the high antidiabetic activity of phenylpropenoic acids. It was proven that
p-methoxycinnamic acid exhibits 100-fold higher activity than the reference compound 1-deoxynojirimycin with documented antidiabetic properties [
32]. The authors of this study explained that such high activity of
p-MCA acid is a result of multidirectional action, which includes stimulation of insulin secretion, improvement of pancreatic
β-cell function, delay of carbohydrate digestion and glucose uptake, inhibition of protein glycation, insulin fibrillation and gluconeogenesis in the liver [
33,
34,
35,
36]. Similar observations regarding the antidiabetic properties of acids were also described by other study authors [
37,
38].
Other in vitro studies demonstrate also the potent hepatoprotective properties of methoxylated derivatives of cinnamic acid in tests performed on rat’s hepatocytes with toxicity induced by carbon tetrachloride (CCl
4). These studies showed that from among tested phenylpropenoic acids, those with the -OCH
3 group in the
para position (such as
p-methoxycinnamic acid and isoferulic acid) exhibit hepatoprotective activity comparable to that of sylibine, even when used in several dozen times-lower concentrations. It has been confirmed that compounds belonging to this group have a direct effect on the activity of liver enzymes and thus positively influence the oxidative stress balance, improve lipid and alcohol metabolism, inhibit inflammation, fibrosis and apoptosis of liver cells [
28]. These results have been further confirmed during in vivo studies conducted on the mice and rat model [
16,
28,
39,
40].
As it turns out, methoxy derivatives of cinnamic acid and their ethyl esters also exhibit significant anti-amnestic properties [
30,
41,
42,
43,
44]. The results of in vivo studies conducted on the mouse model have shown that
p-MCA is able to reduce memory deficits by about 60% in scopolamine-induced amnesia rodents, compared to the control group. It is worth noting that its effect is superior to that of a substance called velnakrine used in clinical trials as a drug in Alzheimer’s disease [
43]. Furthermore, 3,4-dimethoxycinnamic acid was also found to be active in neuroprotective properties. Under in vitro studies in human neuroblastoma cells line (SH-SY5Y), 3,4DMCA strongly bound with prion proteins, reducing the possibility of oligomer formation of these proteins by 30–40% compared to control and significantly increasing the viability of these cells [
44].
The data presented in indicate a number of anticancer properties of methoxy derivatives of cinnamic acid [
45,
46,
47,
48,
49,
50,
51,
52]. Of particular interest seems to be the anticancer activity of
p-MCA acid, which against colon cancer cells (HCT-116) is similar to doxorubicin. At this point, it should also be highlighted that the activity of
p-MCA was more selective against HCT-116 tumor cells than against the normal colon epithelial cell line (NCM460) [
49].
Table 1. Biological potential and mechanism of action of methoxy phenylpropenoic acids.
|
Activity
|
Molecule
|
Research
Model
|
Active
Dose
|
Mechanism
of Action
|
Refs.
|
|
Hepatoprotective
|
FA
|
rats/EtOH
|
20 mg/kg b.w.
|
ALP↓, GGT↓, ALT↓, AST↓
|
[28]
|
|
MFA
|
mice/EtOH
|
5–20 mg/kg b.w.
|
ALT↓, AST↓,
SOD↑, CAT↑, GSH↑
GSH-Px↑, T-AOC↑
|
[40]
|
|
p-MCA
|
rats/CCl4
|
1–5 μM
|
GSH↑, GSH-Px↑, GST↑, GR↑
|
[16]
|
|
p-MCA
|
rats/CCl4
|
50 mg/kg
|
ALP↓, ALT↓, GGT↓
|
[39]
|
|
Antydiabectic
|
p-MCA
|
INS-1 cell line
|
100 μM
|
insulin↑
|
[34]
|
|
p-MCA
|
INS-1 cell line
|
100 μM
|
ions Ca2+↑, insulin↑
|
[35]
|
|
p-MCA
|
Wistar rats
|
5 mg/kg
|
insulin↑
|
[34]
|
|
p-MCA
|
rats/STPZ
|
40 mg/kg
|
insulin↑,
glukogenesis↓
|
[36]
|
|
p-MCA
|
rats/STPZ
|
40–100 mg/kg
|
insulin↑
|
[37]
|
|
p-MCA
|
rats/STPZ
|
10–40 mg/kg
|
Insulin↑
|
[38]
|
|
Neuroprotective
|
FA
|
rats-ROT
|
50 mg/kg m.c.
|
neuron necrosis↓,
IL-1β↓, IL-6↓,
TNF-α↓, CAT↑, SOD↑
|
[30]
|
|
p-MCA
|
rat cortical cells/glutamate
|
1 μM
|
-
|
[41]
|
|
Ep-MCA
|
rat cortical cells/glutamate
|
0.01–1 μM
|
ions Ca2+↑,
glutamatergic
antagonism
|
[42]
|
|
Ep-MCA
|
mice-ICR
|
0.01–2 mg/kg
|
-
|
[43]
|
|
3,4-DMCA
|
SH-SY5Y cell line
|
400 nM (Kd)
|
cell viability↑
|
[44]
|
|
Chemopreventive
and anticancer
|
FA
|
MDA-MB-231 mices
|
100 mg/kg
|
apoptosis↑
metastatic potential↓
|
[23]
|
|
FA
|
mice/UV radiation
|
50 mg/kg
|
DNA protection
|
[45]
|
|
FA
|
PC-3,
LNCaP cell line
|
300 μM
500 μM
|
cell proliferation↓
|
[25]
|
|
FA
|
rats/DMAB
|
40 mg/kg
|
-
|
[46]
|
|
FA
|
rats/4NQO
|
500 ppm
|
-
|
[47]
|
|
p-MCA
|
HepG2 cell line
|
27.1 g/mL (IC50)
|
apoptosis↑
|
[14]
|
|
p-MCA
|
rats
|
40 mg/kg
|
-
|
[48]
|
|
p-MCA
|
HCT-116 cell line
|
10 μM (IC50)
|
apoptosis↓
|
[49]
|
|
Ep-MCA
|
mice/DMAB
|
23.4 mg/kg
|
apoptosis↓
|
[50]
|
|
Ep-MCA
|
MCF-7 cell line
|
360 g/mL (IC50)
|
-
|
[51]
|
|
Ep-MCA
|
rats/DMH
|
40 mg/kg
|
-
|
[52]
|
↓/↑—decrease/increase in activity, - — not known mechanism of action, ALP—alkaline phosphatase, GGT—γ-glutamyl transferase, ALT—alanine transaminase AST—aspartate transaminase, SOD—superoxide dismutase, CAT—catalase, GSH—glutathione, GSH-Px—glutathione peroxidase, T-AOC—total antioxidant capacity, GST—glutathione S-transferase, ROT—rotenone, IL-1β, IL-6—proinflammatory interleukins, TNF-α—tumor necrosis factor, PC-3 and LNCaP—human prostate cancer cell lines, MDA-MB-231, MCF-7—human breast cancer cell lines, HepG2—human liver cancer cell line, HCT-116—human colon cancer cell line DMAB—7,12-dimethylbenz(a)anthracene, STPZ—streptozotocin, 4NQO—nitrohinone oxide, DMH—1,2-dimethylhydrazine.
Summary of biological activities of methoxy derivatives of cinnamic acids like: ferulic, p-methoxy- and 3,4-dimethoxycinnamic acids and their ester derivatives (ferulic acid methyl ester (MFA), p-methoxycinnamic acid ethyl ester (Ep-MCA)) with their mechanisms of action are presented in .
Summing up, it can be concluded that extensive research has demonstrated that the chemical structure of phenylpropenoic acids highly defines the extent of their therapeutic potential. While the presence of the -OH group in the aromatic ring of phenylpropenoic acids is mainly responsible for the occurrence of antioxidant activity, the group -OCH3, especially in the para position relative to the unsaturated side chain, determines their strong antidiabetic as well as hepato- and neuroprotective potential. This proves the high therapeutic potential of this group of compounds both in the prevention and therapy of civilization diseases.
3. Bioavailability of Phenylpropenoic Acids
The therapeutic effect and possibility of the practical application of phytochemicals as therapeutic agents significantly depends on their bioavailability, understood as the concentration in which the compounds reach the general circulation in an unchanged form. This, in the first line, depends on their solubility and permeability through the gastrointestinal tract. One of the main parameters affecting the degree of absorption of cinnamic acid derivatives in the human body is their chemical structure. Kern et al. carried out a study to determine the bioavailability of ferulic acid (FA) from food [
53]. For this purpose, they determined the concentration of this compound that appeared in the blood of volunteers after consumption of a meal consisting of cereal bran, containing 22.5 μM FA per kg of their body weight. The maximum concentration of the compound (expressed as the sum of its metabolites) that was observed in peripheral blood was only 0.2 μM/L 180 min after consumption of the meal. However, the total amount determined in urine, the main route of FA excretion from the body, was about 3% in relation to the amount ingested with food. Such a low content of FA in plasma is caused by its limited absorption from the gastrointestinal tract due to its chemical form of occurrence in natural sources. As it was mentioned earlier, phenolic acids rarely occur in natural sources in the free form. Most of these compounds occur in combinations with mono-, di-, and polysaccharides, sterols, polyamines, glycoproteins, and lignins that form the structure of plant cell walls. Such combinations are not metabolized by human digestive enzymes. Release of free acids and consequently also absorption occurs only as a result of hydrolytic enzymes of intestinal microflora [
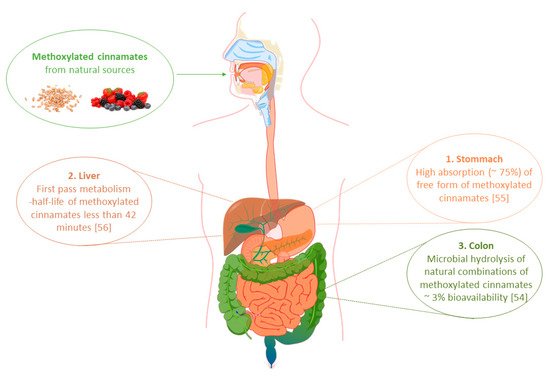
53]. A diagram showing the absorption of dietary methoxy derivatives of phenylpropenoic acids in the human body is presented in .
Figure 2. Absorption of dietary methoxylated derivatives of phenylpropenoic acids in the human gastrointestinal tract.
A relatively higher degree of absorption of phenolic acids can be obtained by their administration in the form of salts or esters of short-chain alcohols. However, even then, the chances of achieving satisfactory concentrations in peripheral blood are still low due to the rapid metabolism of these compounds in the system [
54,
55]. During the studies in rats, it was observed that orally administered free FA is predominantly absorbed from the stomach (74%), from where it reaches the liver through the portal vein. There, as a result of enzymes phase I and II of xenobiotic metabolism, it undergoes sulfonation and conjugation with glucuronic acid to form derivatives with lower biological activity. As a result of such intensive transformations, there is no unchanged form of ferulic acid in the bloodstream and it is almost immediately excreted via urine. Rapid metabolism of ferulic acid was also confirmed in human studies. Orally administrated ferulic acid sodium salt in a dose of 50 mg caused a maximum plasma concentration of 2.5 μmol/L of its unchanged form, observed after 24 min after administration. The calculated half-life of this molecule in the human body was then only 42 min [
55].
The metabolic processes of phenylpropenoic acids also depend on their structure, especially on the number of hydroxy and methoxy substituents present in the aromatic ring. During in vitro studies in human colorectal adenocarcinoma cells (Caco-2), it was proven that 3,4-dimethoxycinnamic acid penetrates the intestinal wall several times easier than the corresponding hydroxy derivative of cinnamic acid [
56]. Moreover, an experiment performed using post-mitochondrial supernatant from mammalian liver cells (S9 fraction) also showed that due to methylation of hydroxy groups, this acid is also characterized by higher metabolic stability [
56]. The presence of -OCH
3 groups inhibits the activity of first-pass liver enzymes responsible for sulfonation and glucuronidation reactions. Therefore, methoxylated derivatives of cinnamic acid reach the bloodstream in unchanged form. However, despite the fact that
O-methylated forms of phenolic acids more easily penetrate into the blood, animal studies show that these compounds are still characterized by a short half-life which is less than 1 h [
57].