Niemann–Pick type C (NPC) disease is an autosomal recessive storage disorder, characterized by abnormal sequestration of unesterified cholesterol in the late endo-lysosomal system of cells. Progressive neurological deterioration and the onset of symptoms, such as ataxia, seizures, cognitive decline, and severe dementia, are pathognomonic features of the disease. In addition, different pathological similarities, including degeneration of hippocampal and cortical neurons, hyperphosphorylated tau, and neurofibrillary tangle formation, have been identified between NPC disease and other neurodegenerative pathologies.
- Niemann–Pick type C
- neurodegeneration
- cognitive decline
- lipid trafficking
- pharmacological treatment
- physical exercise
- nutritional approach
1. Introduction
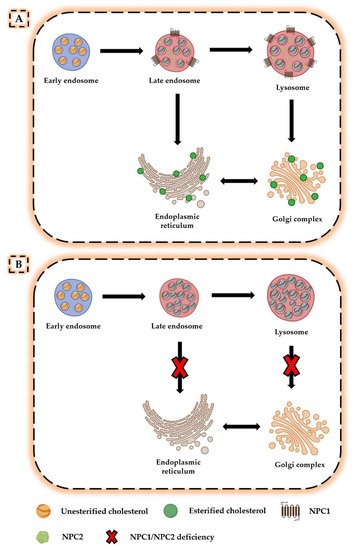
2. Neurodegeneration in NPC Disease
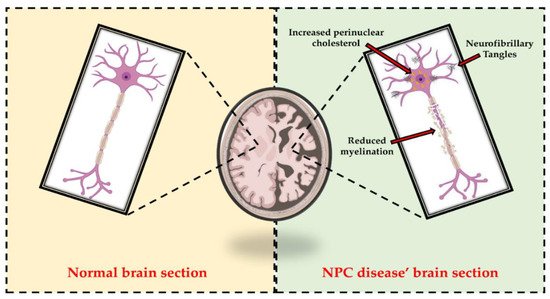
3. Therapeutic Approaches Used to Counteract Cognitive Deficits
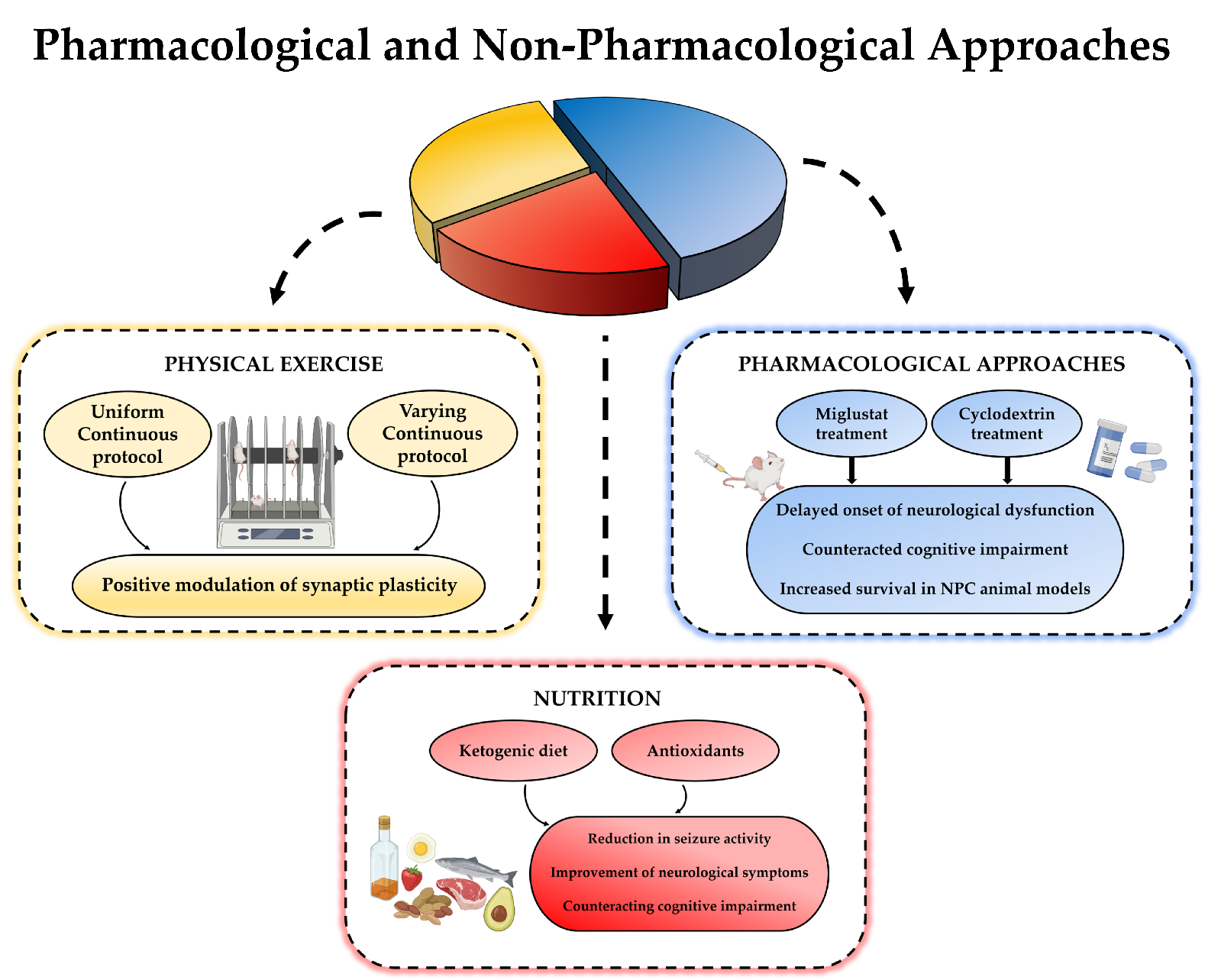
4. Additional Non-Pharmacologic Approaches for Neurodegeneration
4.1. Physical Exercise
4.2. Nutrition
NPC disease.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22126600
References
- Newton, J.; Milstien, S.; Spiegel, S. Niemann-Pick type C disease: The atypical sphingolipidosis. Adv. Biol. Regul. 2018, 70, 82–88.
- Wheeler, S.; Sillence, D.J. Niemann-Pick type C disease: Cellular pathology and pharmacotherapy. J. Neurochem. 2020, 153,674–692. [CrossRef]
- Bi, X.; Liao, G. Cholesterol in Niemann-Pick Type C Disease. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands,2010; Volume 51, pp. 319–335. [CrossRef]
- Lloyd-Evans, E.; Platt, F.M. Lipids on trial: The search for the offending metabolite in Niemann-Pick type C disease. Traffic 2010,11, 419–428. [CrossRef]
- Newton, J.; Hait, N.C.; Maceyka, M.; Colaco, A.; Maczis, M.; Wassif, C.A.; Cougnoux, A.; Porter, F.D.; Milstien, S.; Platt, N.;et al. FTY720/fingolimod increases NPC1 and NPC2 expression and reduces cholesterol and sphingolipid accumulation in Niemann-Pick type C mutant fibroblasts. FASEB J. 2017, 31, 1719–1730. [CrossRef]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.L.; Taylor,R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis.2010, 69, 1502–1510. [CrossRef]
- Héron, B.; Valayannopoulos, V.; Baruteau, J.; Chabrol, B.; Ogier, H.; Latour, P.; Dobbelaere, D.; Eyer, D.; Labarthe, F.; Maurey, H.;et al. Miglustat therapy in the French cohort of paediatric patients with Niemann-Pick disease type C. Orphanet J. Rare Dis. 2012,7, 36. [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNSsynaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [CrossRef]
- Pagano, R.E. Endocytic trafficking of glycosphingolipids in sphingolipid storage diseases. Philos. Trans. R. Soc. Lond. Ser. B Biol.Sci. 2003, 358, 885–891. [CrossRef] [PubMed]
- Wang, M.L.; Motamed, M.; Infante, R.E.; Abi-Mosleh, L.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Identification of surface residues on Niemann-Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010, 12, 166–173.
- Xie, X.; Brown, M.S.; Shelton, J.M.; Richardson, J.A.; Goldstein, J.L.; Liang, G. Amino acid substitution in NPC1 that abolishes cholesterol binding reproduces phenotype of complete NPC1 deficiency in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 15330–15335.
- Wojtanik, K.M.; Liscum, L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J. Biol. Chem. 2003, 278, 14850–14856. [CrossRef]
- Berzina, Z.; Solanko, L.M.; Mehadi, A.S.; Jensen, M.L.V.; Lund, F.W.; Modzel, M.; Szomek, M.; Solanko, K.A.; Dupont, A.; Nielsen,G.K.; et al. Niemann-Pick C2 protein regulates sterol transport between plasma membrane and late endosomes in human fibroblasts. Chem. Phys. Lipids 2018, 213, 48–61. [CrossRef] [PubMed]
- Hodošˇcek, M.; Elghobashi-Meinhardt, N. Simulations of NPC1(NTD):NPC2 Protein Complex Reveal Cholesterol Transfer Pathways. Int. J. Mol. Sci. 2018, 19, 2623. [CrossRef] [PubMed]
- Winkler, M.B.L.; Kidmose, R.T.; Szomek, M.; Thaysen, K.; Rawson, S.; Muench, S.P.; Wüstner, D.; Pedersen, B.P. Structural Insight into Eukaryotic Sterol Transport through Niemann-Pick Type C Proteins. Cell 2019, 179, 485–497.e18. [CrossRef]
- Elghobashi-Meinhardt, N. Computational Tools Unravel Putative Sterol Binding Sites in the Lysosomal NPC1 Protein. J. Chem. Inf. Model. 2019, 59, 2432–2441. [CrossRef]
- Li, X.; Wang, J.; Coutavas, E.; Shi, H.; Hao, Q.; Blobel, G. Structure of human Niemann-Pick C1 protein. Proc. Natl. Acad. Sci. USA 2016, 113, 8212–8217. [CrossRef] [PubMed]
- Wheeler, S.; Schmid, R.; Sillence, D.J. Lipid–Protein Interactions in Niemann–Pick Type C Disease: Insights from Molecular Modeling. Int. J. Mol. Sci. 2019, 20, 717. [CrossRef] [PubMed]
- Vanier, M.T.; Millat, G. Niemann-Pick disease type C. Clin. Genet. 2003, 64, 269–281. [CrossRef]
- Reddy, J.V.; Ganley, I.G.; Pfeffer, S.R. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS ONE 2006, 1, e19. [CrossRef] [PubMed]
- Liscum, L.; Faust, J.R. Low density lipoprotein (LDL)-mediated suppression of cholesterol synthesis and LDL uptake is defective in Niemann-Pick type C fibroblasts. J. Biol. Chem. 1987, 262, 17002–17008.
- Olkkonen, V.M.; Levine, T.P. Oxysterol binding proteins: In more than one place at one time? Biochem. Cell Biol. 2004, 82, 87–98.
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008, 14, 1247–1255. [CrossRef] [PubMed]
- Lloyd-Evans, E.; Platt, F.M. Lysosomal Ca(2+) homeostasis: Role in pathogenesis of lysosomal storage diseases. Cell Calcium 2011, 50, 200–205. [CrossRef] [PubMed]
- Kågedal, K.; Zhao, M.; Svensson, I.; Brunk, U.T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 2001, 359, 335–343. [CrossRef] [PubMed]
- Patterson, M.C.; Hendriksz, C.J.; Walterfang, M.; Sedel, F.; Vanier, M.T.; Wijburg, F. Recommendations for the diagnosis and management of Niemann-Pick disease type C: An update. Mol. Genet. Metab. 2012, 106, 330–344. [CrossRef] [PubMed]
- Spiegel, R.; Raas-Rothschild, A.; Reish, O.; Regev, M.; Meiner, V.; Bargal, R.; Sury, V.; Meir, K.; Nadjari, M.; Hermann, G.; et al. The clinical spectrum of fetal Niemann-Pick type C. Am. J. Med. Genet. A 2009, 149A, 446–450. [CrossRef]
- Trendelenburg, G.; Vanier, M.T.; Maza, S.; Millat, G.; Bohner, G.; Munz, D.L.; Zschenderlein, R. Niemann-Pick type C disease in a 68-year-old patient. J. Neurol. Neurosurg. Psychiatry 2006, 77, 997–998.
- Sévin, M.; Lesca, G.; Baumann, N.; Millat, G.; Lyon-Caen, O.; Vanier, M.T.; Sedel, F. The adult form of Niemann-Pick disease type C. Brain 2007, 130, 120–133. [CrossRef] [PubMed]
- Garver, W.S.; Francis, G.A.; Jelinek, D.; Shepherd, G.; Flynn, J.; Castro, G.; Walsh Vockley, C.; Coppock, D.L.; Pettit, K.M.;Heidenreich, R.A.; et al. The National Niemann-Pick C1 disease database: Report of clinical features and health problems. Am. J.Med. Genet. A 2007, 143A, 1204–1211. [CrossRef]
- Vanier, M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010, 5, 16. [CrossRef] [PubMed]
- Wassif, C.A.; Cross, J.L.; Iben, J.; Sanchez-Pulido, L.; Cougnoux, A.; Platt, F.M.; Ory, D.S.; Ponting, C.P.; Bailey-Wilson, J.E.;Biesecker, L.G.; et al. High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predictedby analysis of massively parallel sequencing data sets. Genet. Med. 2016, 18, 41–48. [CrossRef]
- Arenas, F.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Intracellular Cholesterol Trafficking and Impact in Neurodegeneration. Front.Mol. Neurosci. 2017, 10, 382. [CrossRef] [PubMed]
- Schultz, M.L.; Krus, K.L.; Lieberman, A.P. Lysosome and endoplasmic reticulum quality control pathways in Niemann-Pick type C disease. Brain Res. 2016, 1649, 181–188. [CrossRef] [PubMed]
- Malnar, M.; Hecimovic, S.; Mattsson, N.; Zetterberg, H. Bidirectional links between Alzheimer’s disease and Niemann-Pick type C disease. Neurobiol. Dis. 2014, 72, 37–47. [CrossRef]
- Elrick, M.J.; Pacheco, C.D.; Yu, T.; Dadgar, N.; Shakkottai, V.G.;Ware, C.; Paulson, H.L.; Lieberman, A.P. Conditional Niemann-Pick C mice demonstrate cell autonomous Purkinje cell neurodegeneration. Hum. Mol. Genet. 2010, 19, 837–847. [CrossRef]
- Yu, T.; Shakkottai, V.G.; Chung, C.; Lieberman, A.P. Temporal and cell-specific deletion establishes that neuronal Npc1 deficiency is sufficient to mediate neurodegeneration. Hum. Mol. Genet. 2011, 20, 4440–4451. [CrossRef]
- Lopez, M.E.; Klein, A.D.; Dimbil, U.J.; Scott, M.P. Anatomically defined neuron-based rescue of neurodegenerative Niemann-Picktype C disorder. J. Neurosci. 2011, 31, 4367–4378. [CrossRef] [PubMed]
- Yu, W.; Gong, J.-S.; Ko, M.; Garver, W.S.; Yanagisawa, K.; Michikawa, M. Altered cholesterol metabolism in Niemann-Pick typeC1 mouse brains affects mitochondrial function. J. Biol. Chem. 2005, 280, 11731–11739. [CrossRef] [PubMed]
- Fernández, A.; Llacuna, L.; Fernández-Checa, J.C.; Colell, A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J. Neurosci. 2009, 29, 6394–6405. [CrossRef]
- Benussi, A.; Cotelli, M.S.; Padovani, A.; Borroni, B. Recent neuroimaging, neurophysiological, and neuropathological advances for the understanding of NPC. F1000Research 2018, 7, 194. [CrossRef]
- Love, S.; Bridges, L.R.; Case, C.P. Neurofibrillary tangles in Niemann-Pick disease type C. Brain 1995, 118, 119–129. [CrossRef]
- Malnar, M.; Kosicek, M.; Mitterreiter, S.; Omerbasic, D.; Lichtenthaler, S.F.; Goate, A.; Hecimovic, S. Niemann-Pick type C cells show cholesterol dependent decrease of APP expression at the cell surface and its increased processing through the beta-secretase pathway. Biochim. Biophys. Acta 2010, 1802, 682–691.
- Yamazaki, T.; Chang, T.Y.; Haass, C.; Ihara, Y. Accumulation and aggregation of amyloid beta-protein in late endosomes of Niemann-pick type C cells. J. Biol. Chem. 2001, 276, 4454–4460. [CrossRef]
- Runz, H.; Rietdorf, J.; Tomic, I.; de Bernard, M.; Beyreuther, K.; Pepperkok, R.; Hartmann, T. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J. Neurosci. 2002, 22, 1679–1689. [CrossRef] [PubMed]
- Jin, L.-W.; Shie, F.-S.; Maezawa, I.; Vincent, I.; Bird, T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am. J. Pathol. 2004,164, 975–985. [CrossRef]
- Burns, M.; Gaynor, K.; Olm, V.; Mercken, M.; LaFrancois, J.; Wang, L.; Mathews, P.M.; Noble, W.; Matsuoka, Y.; Duff, K. Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J. Neurosci. 2003, 23,5645–5649. [CrossRef] [PubMed]
- Guénette, S.Y.; Chen, J.; Jondro, P.D.; Tanzi, R.E. Association of a novel human FE65-like protein with the cytoplasmic domain of the beta-amyloid precursor protein. Proc. Natl. Acad. Sci. USA 1996, 93, 10832–10837. [CrossRef]
- Chang, Y.; Tesco, G.; Jeong, W.J.; Lindsley, L.; Eckman, E.A.; Eckman, C.B.; Tanzi, R.E.; Guénette, S.Y. Generation of the betaamyloid peptide and the amyloid precursor protein C-terminal fragment gamma are potentiated by FE65L1. J. Biol. Chem. 2003,278, 51100–51107. [CrossRef] [PubMed]
- Kågedal, K.; Kim, W.S.; Appelqvist, H.; Chan, S.; Cheng, D.; Agholme, L.; Barnham, K.; McCann, H.; Halliday, G.; Garner, B. Increased expression of the lysosomal cholesterol transporter NPC1 in Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801,831–838. [CrossRef]
- Yu, T.; Lieberman, A.P. Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin. PLoS Genet. 2013, 9, e1003462. [CrossRef]
- Takikita, S.; Fukuda, T.; Mohri, I.; Yagi, T.; Suzuki, K. Perturbed myelination process of premyelinating oligodendrocyte in Niemann-Pick type C mouse. J. Neuropathol. Exp. Neurol. 2004, 63, 660–673. [CrossRef] [PubMed]
- Bowman, E.A.; Velakoulis, D.; Desmond, P.; Walterfang, M. Longitudinal Changes in White Matter Fractional Anisotropy in Adult-Onset Niemann-Pick Disease Type C Patients Treated with Miglustat. JIMD Rep. 2018, 39, 39–43. [CrossRef] [PubMed]
- Masingue, M.; Adanyeguh, I.; Nadjar, Y.; Sedel, F.; Galanaud, D.; Mochel, F. Evolution of structural neuroimaging biomarkers in a series of adult patients with Niemann-Pick type C under treatment. Orphanet J. Rare Dis. 2017, 12, 22. [CrossRef]
- Rego, T.; Farrand, S.; Goh, A.M.Y.; Eratne, D.; Kelso, W.; Mangelsdorf, S.; Velakoulis, D.; Walterfang, M. Psychiatric and Cognitive Symptoms Associated with Niemann-Pick Type C Disease: Neurobiology and Management. CNS Drugs 2019, 33, 125–142.
- Crocker, A.C.; Farber, S. Niemann-Pick disease: A review of eighteen patients. Medicine (Baltimore) 1958, 37, 1–95. [CrossRef]
- Sylvain, M.; Arnold, D.L.; Scriver, C.R.; Schreiber, R.; Shevell, M.I. Magnetic resonance spectroscopy in Niemann-Pick disease type C: Correlation with diagnosis and clinical response to cholestyramine and lovastatin. Pediatr. Neurol. 1994, 10, 228–232.
- Patterson, M.C.; Di Bisceglie, A.M.; Higgins, J.J.; Abel, R.B.; Schiffmann, R.; Parker, C.C.; Argoff, C.E.; Grewal, R.P.; Yu, K.;Pentchev, P.G. The effect of cholesterol-lowering agents on hepatic and plasma cholesterol in Niemann-Pick disease type C.Neurology 1993, 43, 61–64. [CrossRef] [PubMed]
- Pineda, M.; Walterfang, M.; Patterson, M.C. Miglustat in Niemann-Pick disease type C patients: A review. Orphanet J. Rare Dis.
- Erickson, R.P.; Garver, W.S.; Camargo, F.; Hossain, G.S.; Heidenreich, R.A. Pharmacological and genetic modifications of somatic cholesterol do not substantially alter the course of CNS disease in Niemann-Pick C mice. J. Inherit. Metab. Dis. 2000, 23, 54–62.
- Beheregaray, A.P.C.; Souza, F.T.S.; Coelho, J.C. Effect of a peroxysomal proliferator agent on intracellular cholesterol accumulation in cultured fibroblasts from Niemann-Pick type C disease patients. Clin. Chim. Acta 2003, 336, 137–142. [CrossRef]2018, 13, 140. [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.;Powell, K.E. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci.Sports Exerc. 2019, 51, 1242–1251. [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence.Psychol. Med. 2009, 39, 3–11. [CrossRef] [PubMed]
- Liu, Y.; Yan, T.; Chu, J.M.-T.; Chen, Y.; Dunnett, S.; Ho, Y.-S.; Wong, G.T.-C.; Chang, R.C.-C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Invest. 2019, 99, 943–957.
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes (Basel) 2019, 10, 720. [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392.
- Dahlin, E.; Andersson, M.; Thorén, A.; Hanse, E.; Seth, H. Effects of physical exercise and stress on hippocampal CA1 and dentate gyrus synaptic transmission and long-term potentiation in adolescent and adult Wistar rats. Neuroscience 2019, 408, 22–30.
- Brown, J.; Cooper-Kuhn, C.M.; Kempermann, G.; Van Praag, H.; Winkler, J.; Gage, F.H.; Kuhn, H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003, 17, 2042–2046. [CrossRef]
- Farmer, J.; Zhao, X.; van Praag, H.; Wodtke, K.; Gage, F.H.; Christie, B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004, 124, 71–79. [CrossRef]
- Van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J.Neurosci. 2005, 25, 8680–8685. [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022.
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [CrossRef]
- Palmieri, M.; Cariati, I.; Scimeca, M.; Pallone, G.; Bonanno, E.; Tancredi, V.; D’Arcangelo, G.; Frank, C. Effects of short-term aerobic exercise in a mouse model of Niemann-Pick type C disease on synaptic and muscle plasticity. Ann. Ist. Super. Sanita 2019, 55. [CrossRef]
- Cariati, I.; Scimeca, M.; Tancredi, V.; D’Amico, A.G.; Pallone, G.; Palmieri, M.; Frank, C.; D’Arcangelo, G. Effects of different continuous aerobic training protocols in a heterozygous mouse model of Niemann-Pick type C disease. J. Funct. Morphol. Kinesiol. 2020, 5, 53. [CrossRef] [PubMed]
- Pallone, G.; Palmieri, M.; Cariati, I.; Bei, R.; Masuelli, L.; D’Arcangelo, G.; Tancredi, V. Different continuous training modalities result in distinctive effects on muscle structure, plasticity and function. Biomed. Rep. 2020, 12. [CrossRef]
- Scholl-Bürgi, S.; Höller, A.; Pichler, K.; Michel, M.; Haberlandt, E.; Karall, D. Ketogenic diets in patients with inherited metabolic disorders. J. Inherit. Metab. Dis. 2015, 38, 765–773. [CrossRef] [PubMed]
- Höller, A.; Albrecht, U.; Baumgartner Sigl, S.; Zöggeler, T.; Ramoser, G.; Bernar, B.; Karall, D.; Scholl-Bürgi, S. Successful implementation of classical ketogenic dietary therapy in a patient with Niemann-Pick disease type C. Mol. Genet. Metab. Rep. 2021, 27, 100723. [CrossRef]
- Marín, T.; Contreras, P.; Castro, J.F.; Chamorro, D.; Balboa, E.; Bosch-Morató, M.; Muñoz, F.J.; Alvarez, A.R.; Zanlungo, S. Vitamin E dietary supplementation improves neurological symptoms and decreases c-Abl/p73 activation in Niemann-Pick C mice. Nutrients 2014, 6, 3000–3017. [CrossRef] [PubMed]
- Marshall, C.A.; Borbon, I.A.; Erickson, R.P. Relative efficacy of nicotinamide treatment of a mouse model of infantile Niemann-Pick C1 disease. J. Appl. Genet. 2017, 58, 99–102. [CrossRef]
