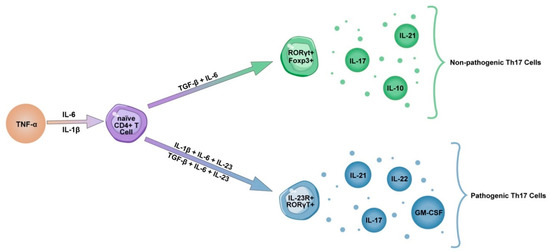
Increasing evidence suggests that the Th17 inflammatory response plays an important role in the pathogenesis of COVID-19 pneumonia. Exacerbation of the immune response occurs through the release of cytokines such as IL-17 and GM-CSF, the promotion of neutrophil migration and the downregulation of the Treg response. Unlike Th17 cells, Treg cells express anti-inflammatory mediators (IL-4, IL-10 and TGF-β) and play an important role in weakening overactive immune responses [
35]. The Treg/Th17 cell ratio is decreased in patients with severe COVID-19 due to the decreased number of Treg cells, indicating the insufficient regulation of pro-inflammatory responses [
58,
59]. Furthermore, prior evidence shows that the Treg/Th17 balance is associated with the severity of uncontrolled systemic inflammation in Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) [
60,
61]. Therefore, the dysregulation of the Treg/Th17 cells ratio skewing towards the Th17 phenotype may contribute to the uncontrolled release of cytokine and chemokine cascades in COVID-19 patients, leading to aggravated inflammatory responses and tissue damage [
35]. Several studies demonstrated increased levels of IL-17 and GM-CSF in peripheral blood and tears of patients with COVID-19, and a higher fraction of Th17 cells in bronchoalveolar lavage fluid of these patients [
11,
62,
63,
64,
65,
66,
67,
68,
69]. Similarly, robust Th17 responses were observed in patients with MERS-CoV and SARS-CoV infections [
70,
71,
72]. A strong Th17 response was also observed in H1N1 influenza virus infection [
73]; moreover, prior evidence associated IL-17 with Acute Respiratory Distress Syndrome and Neonatal Respiratory Distress Syndrome (NRDS) [
74,
75]. Increased concentrations of IL-17 were found in plasma and alveolar fluid of patients with ARDS. In addition, when compared to survivors, significantly higher levels of IL-17 were found in a group of non-survivors. Furthermore, a negative correlation between the PaO
2/FiO
2 ratio and level of IL-17 was found in these patients [
76]. Interestingly, it has been shown that in macaques infected with the simian immunodeficiency virus, the percentage of CD161
+CD8
+ Tc17 cells producing IL-17 in lung tissue was four times higher than in peripheral blood. Additionally, these cells could secrete more IL-17 than those present in peripheral blood [
77]. Thus, IL-17 might promote pulmonary inflammation, following the infection by neutrophil and monocyte migration to the lungs, and by activating other cytokine cascades (G-CSF, TNFα, IL-1β and IL-6) [
11,
78,
79]. In addition, plasma from COVID-19 patients revealed a fourfold increase of the IFN-γ levels, which activates macrophages to produce proinflammatory cytokines, indicating a Th1/Th17 response [
11,
80,
81]. Elevated levels of IFN-γ were also found in MERS-CoV infections [
82]. Moreover, prior evidence shows that high concentrations of IFN-γ in rapidly progressive interstitial lung disease associated with dermatomyositis positively correlated with the ground-glass opacity score (G-score) in CT [
51].
Taken together, the evidence supports the involvement of a Th17 mediated response in the pathogenesis of pneumonia caused by SARS-CoV-2. Therefore, targeting the Th17 phenotype might be beneficial in patients with a dominant Th17 response.