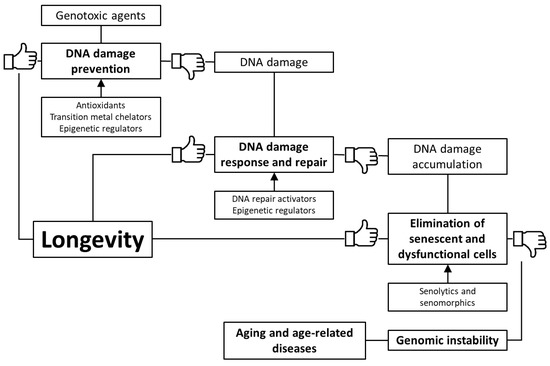
Throughout life, organisms are exposed to various exogenous and endogenous factors that cause DNA damages and somatic mutations provoking genomic instability. At a young age, compensatory mechanisms of genome protection are activated to prevent phenotypic and functional changes. However, the increasing stress and age-related deterioration in the functioning of these mechanisms result in damage accumulation, overcoming the functional threshold. In the tissues of aging animals and humans, the frequency of DNA damage and somatic mutations increases, the genome instability appears, which is manifested in a surge of point mutations, breaks and cross-linking of DNA strands, transpositions and translocations, aneuploidies. This leads to aging and the development of age-related diseases. There are several ways to counteract these changes: (1) prevention of DNA damage through stimulation of antioxidant and detoxification systems, as well as transition metal chelation; (2) regulation of DNA methylation, chromatin structure, non-coding RNA activity and prevention of nuclear architecture alterations; (3) improving DNA damage response and repair; (4) selective removal of damaged non-functional and senescent cells. Fortunately, there are a number of trace elements, vitamins, polyphenols, terpenes, polyamines, and other phytochemicals, as well as a number of synthetic pharmacological substances, that have genome-protective and geroprotective effects. Some of them are cofactors of antioxidant enzymes, DNA repair, or epigenetic regulation enzymes (in particular, Zn, Cu, Mg, NAD+, vitamin C, vitamin A, butyrate, glutathione). Others have free radical and advanced glycation endproduct scavenging, anti-inflammatory, heavy metal chelator effects preventing oxidative DNA damages, DNA adduct formation, as well as reducing DNA breaks and cross-linking. More promising compounds targeted on epigenetic mechanisms or stimulate pathways of DNA damage response and repair. Currently, the clinical effectiveness of their application for geroprotection and possible side effects are not clear enough and require future investigation. Unfortunately, most substances have a non-selective effect and are often conditioned by hormesis, a non-selective stress response. Furthermore, they require adjuvant therapy. Additionally, senolytics and senomorphics may be useful to eliminate or prevent the accumulation of harmful cells in an organism. However, they also need additional conditions, in particular, sufficient regenerative potential to be replaced by functional cells. Their effect is more selective but is associated with a number of side effects. For example, they can induce apoptosis of normal cells or promote the proliferation of tumor cells, increase their survival during therapy, or promote metastasis. Consequently, the development of selective drugs or complex therapy targeted on maintaining the genome integrity and its coordinated functioning could become an advanced direction of gerontology and pharmacology.
Introduction
The accumulation of genome damage and somatic mutations leading to genome instability are important determinants and hallmarks of aging [
1,
2,
3]. Somatic mutagenesis as a key mechanism of aging was proposed by Leo Szilard in 1959 [
4]. At the same time, recent theories also explain the nature of aging by impairments in maintaining the genome functioning stability (particularly, somatic mutation catastrophe theory) [
5].
The consequences of the failure of mechanisms to maintain genome stability are vividly illustrated by the pathological patterns of numerous accelerated asging syndromes that are caused by mutations in DNA repair genes (for example, Werner, Cocaine, Bloom syndromes, xeroderma pigmentosum, ataxia-telangiectasia, and others) and nuclear architecture maintenance genes (laminopathy, in particular, Hutchinson–Gilford syndrome) [
6,
7,
8,
9,
10]. On the other hand, an increased expression of a number of genes, providing a response to DNA damage and repair, causes an increase in the lifespan of model animals [
2,
11]. Species with extreme longevity, such as naked mole rats, Brandt bats, whales, mole rat
Spalax, and parrots have adaptive features of repair mechanisms that increase the stability of their DNA [
12,
13,
14,
15,
16]. In addition, reliable DNA protection is one of the reasons for the immortality of germline cells [
17]. Genome instability accompanies age-related diseases such as cancer, heart failure, type 2 diabetes, chronic obstructive pulmonary disease, stroke, Alzheimer’s disease and Parkinson’s disease, chronic kidney disease, atherosclerosis, osteoporosis, sarcopenia [
7,
18].
Based on the foregoing, we suggest that stimulation of genome defense mechanisms may be a promising strategy to increase the lifespan and prevent the development of age-related diseases. There are several ways to achieve this goal: (1) prevention of DNA damage through stimulation of antioxidant and detoxification systems, as well as transition metal chelation; (2) regulation of DNA methylation, chromatin structure, non-coding RNA activity and prevention of nuclear architecture alterations; (3) improving DNA damage response and repair; (4) selective removal of non-functional and senescent cells (). In the article, we have reviewed data about the genome-protecting effects of various trace elements, vitamins, polyphenols, terpenes, and other phytochemicals, as well as a number of synthetic pharmacological substances.
Figure 1. Key mechanisms of genome protection by pharmacological interventions.
Conclusions
The aging process is accompanied by a progressive accumulation of DNA damages, epigenetic ‘DNA scars’, somatic mutations, and epimutations that provoke genomic instability. These changes cause disturbances in the activity of vital genes, disruption of cellular metabolism, and cellular senescence. As a result, dysfunctional cells accumulate in organs and tissues of an organism, inducing chronic inflammation, functional and metabolic deterioration, and the regenerative potential decreases, which condition the development of the aging process itself and risk of aging-related diseases. Preservation of the genetic stability of stem cells, which otherwise may cause aberrant differentiation or become tumor stem cells, is especially important.
Fortunately, there are a number of trace elements, vitamins, polyphenols, terpenes, polyamines, and other phytochemicals, as well as a number of synthetic pharmacological substances, that have genome-protective and geroprotective effects. Some of them are cofactors of antioxidant enzymes, DNA repair, or epigenetic regulation enzymes (in particular, Zn, Cu, Mg, NAD+, vitamin C, vitamin A, butyrate, glutathione). Others have free radical and advanced glycation endproduct scavenging, anti-inflammatory, heavy metal chelator effects preventing oxidative DNA damages, DNA adduct formation, as well as reducing DNA breaks and cross-linking. More promising compounds targeted on epigenetic mechanisms or stimulate pathways of DNA damage response and repair. Currently, the clinical effectiveness of their application for geroprotection and possible side effects are not clear enough and require future investigation. Unfortunately, most substances have a non-selective effect and are often conditioned by hormesis, a non-selective stress response. Furthermore, they require adjuvant therapy. Additionally, senolytics and senomorphics may be useful to eliminate or prevent the accumulation of harmful cells in an organism. However, they also need additional conditions, in particular, sufficient regenerative potential to be replaced by functional cells. Their effect is more selective but is associated with a number of side effects. For example, they can induce apoptosis of normal cells or promote the proliferation of tumor cells, increase their survival during therapy, or promote metastasis.
Consequently, the development of selective drugs or complex therapy targeted on maintaining the genome integrity and its coordinated functioning could become an advanced direction of gerontology and pharmacology.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21124484