Melanoma is the most lethal form of skin cancer. Melanoma is usually curable with surgery if detected early, however, treatment options for patients with metastatic melanoma are limited and the five-year survival rate for metastatic melanoma had been 15–20% before the advent of immunotherapy. Treatment with immune checkpoint inhibitors has increased long-term survival outcomes in patients with advanced melanoma to as high as 50% although individual response can vary greatly. A mutation within the MAPK pathway leads to uncontrollable growth and ultimately develops into cancer. The most common driver mutation that leads to this characteristic overactivation in the MAPK pathway is the B-RAF mutation. Current combinations of BRAF and MEK inhibitors that have demonstrated improved patient outcomes include dabrafenib with trametinib, vemurafenib with cobimetinib or encorafenib with binimetinib. Treatment with BRAF and MEK inhibitors has met challenges as patient responses began to drop due to the development of resistance to these inhibitors which paved the way for development of immunotherapies and other small molecule inhibitor approaches to address this. Resistance to these inhibitors continues to push the need to expand our understanding of novel mechanisms of resistance associated with treatment therapies.
1. Introduction
Melanoma is the uncontrollable division of melanocytes located within the deep layer of the epidermis [
1]. Although invasive melanoma is the third most common type of skin cancer, it is the most serious compared to its other two counterparts- basal cell carcinoma and squamous cell carcinoma. The American Cancer Society estimates that in 2019 there will be 96,480 new cases of melanoma diagnosed accompanied by 7,230 expected deaths. The five-year survival rate for metastatic melanoma has been 15–20% [
2], although these statistics are rapidly improving with the success of immune checkpoint inhibitors. Treatment with immune checkpoint inhibitors demonstrated substantial clinical efficacy along with long-term survival outcomes in patients with advanced melanoma [
3,
4,
5].
An important risk factor for melanoma is exposure to direct and chronic ultraviolet (UV) radiation which, increases the risk for DNA damage and leads to genetic changes. Familial history is also one of the risk factors for developing melanoma, with cyclin-dependent kinase inhibitor 2A (CDKN2A) and cyclin-dependent kinase 4 (CDK4) being the most common heritable mutations [
1]. Germ-line polymorphisms of the melanocortin-1 receptor (MC1R) also confers susceptibility due to its ability to control the level of skin pigmentation in response to UV radiation [
2,
6].
The molecular changes occurring in the progression of melanoma serve as points for therapeutic interference. Typically, the preliminary change of a melanocyte into a benign nevus remains controlled and is non-cancerous. However, some molecular changes can lead to overactivation of growth regulatory pathways, such as the mitogen-activated protein kinase (MAPK) signaling pathway [
2]. The MAPK pathway is crucial in relaying extracellular signals in order to keep a balance of growth/proliferation and apoptosis within the cell. A mutation within MAPK pathway leads to uncontrollable growth and ultimately develops into cancer [
7]. The most common driver mutation that leads to this characteristic overactivation in the MAPK pathway is the B-RAF mutation [
2]. Raf is a family of oncogenic serine-threonine protein kinases within the MAPK pathway with three isoforms: A-RAF, B-RAF, and C-RAF [
7,
8]. B-RAF-mutant melanoma accounts for nearly 50% of metastatic melanoma cases. Substitution of valine (V) for glutamic acid (E) at amino acid position 600 (V600E) represents 84.6% of the B-RAF mutations. A second common substitution of valine (V) for lysine (K) at amino acid position 600 (V600K), representating 7.7% of the B-RAF mutations [
9]. B-RAF mutant melanoma is typically found in younger patients and is characterized by a superficial spreading tumor or nodular tumor that can be found in areas without chronic sun exposure. It has a higher chance of metastasizing into brain along with a shorter survival time as compared to the non-BRAF mutant melanoma. A B-RAF mutation alone may not contribute to the development of melanoma; but accompanying driver mutations in tumor suppressor genes are commonly indispensable leading to the development of malignant melanoma [
9].
Several studies have addressed the need for molecular testing with respect to B-RAF mutations in order to tailor the best course of treatment available for each patient. [
9,
10]. Treatment options include surgery, immunotherapy, targeted therapy, chemotherapy, inclusion in a clinical trial and radiation [
11]. Stage I and II melanoma can typically be surgically excised in concert with a sentinel lymph node biopsy if there is concern for metastasis. Stage III and IV melanoma require more systemic interventions due to the aggressiveness of the tumor and increased tumor burden [
8]. The current standard of care for a patient diagnosed with B-RAF mutant metastatic melanoma is to first consider eligibility for a metastasectomy. Regardless of qualifications for the metastasectomy, the next step is to design a treatment regimen with either checkpoint inhibition immunotherapy or a molecularly targeted therapy [
9,
10].
Current targeted therapies include a combination of B-RAF and MEK inhibitors. Vemurafenib was the first FDA-approved B-RAF inhibitor in 2011, followed by approval of dabrafenib in 2013 [
8]. The most recent FDA-approved B-RAF inhibitor is encorafenib, approved in 2018 [
12]. In parallel with the discovery and use of B-RAF inhibitors opened up the avenue for development of MEK inhibitors, targeting a molecule downstream of the B-RAF protein. The first MEK-inhibitor, trametinib, was approved by FDA in 2013, followed by approval of cobimetinib in 2015 [
8]. Another recently FDA-approved MEK inhibitor was binimetinib [
12]. Current combinations of BRAF and MEK inhibitors that have demonstrated improved patient outcomes include dabrafenib with trametinib, vemurafenib with cobimetinib or encorafenib with binimetinib [
13]. Treatment with BRAF and MEK inhibitors has met with some challenges as patient responses began to drop due to the development of resistance to these inhibitors which paved the way for development of immunotherapies and other small molecule inhibitor approaches to address this.
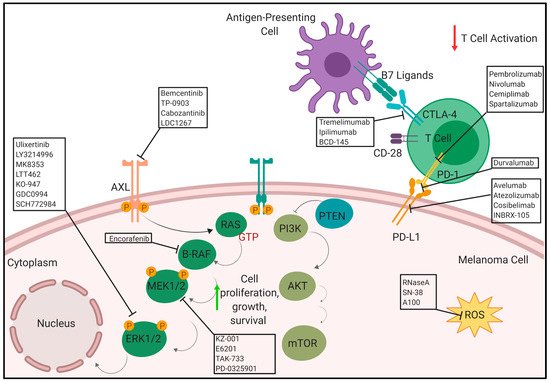
Current immunotherapies include the anti-cytotoxic T-lymphocyte antigen 4 antibody (anti-CTLA-4) and two anti-programmed death protein 1 antibodies (anti-PD1) [
8]. Current preclinical and clinical trials are underway to determine the efficacy and benefits of combining immunotherapy treatment regimen alone or in combination with BRAF and MEK inhibitors for treatment of patients with BRAF mutant melanoma [
13,
14,
15,
16]. New avenues exploring the possible combination therapies of BRAF/MEK inhibitors with immunotherapy drugs are being tested. Combination therapies are not only limited to MAPK pathway targeted therapies plus immunotherapy but have expanded to include other molecules such as AXL and ROS that have a role in the development of drug resistance. These have emerged as alternative treatment options for treating metastatic melanoma patients. Preclinical and clinical trials evaluating the efficacy of various PI3K and CDK4/6 inhibitors in combination with BRAF and MEK inhibitors are also initiated [
17,
18,
19,
20,
21,
22].
2. Therapies
summarizes the therapies in pre-clinical and clinical phases, described in this review to treat patients with metastatic melanoma.
Figure 1. Novel therapies in pre-clinical and clinical phases (Anti PD-1/anti-PD-L1, anti CTLA-4, AXL inhibitors. BRAF inhibitors. ERK inhibitors and ROS activated prodrugs) to treat patients with metastatic melanoma (created with BioRender;
www.biorender.com).
3. Anti-PD-1/PD-L1
The immunogenic nature of melanoma was utilized to develop several immunotherapeutic treatment strategies especially with regards to the programmed cell death (PD-1) receptor and its ligand, PD-L1. Antibodies targeting the PD-1 axis has shown significant promise in the clinic for treatment of metastatic melanoma either as a monotherapy or in combination with Ipilimumab. There are several ongoing clinical trials using anti-PD1 and anti-PD-L1 antibodies. Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on the surface of the cancer cells that inhibits the immune system via suppressing the T-cell activity. Anti-PD-1 monoclonal antibodies block the PD-1 receptor which maintains T-cells in activated state to suppress the tumor growth [
78]. There are several anti-PD-1/PD-L1 monoclonal antibodies including pembrolizumab (Keytruda
®), nivolumab (Opdivo
®), avelumab (Bavencio
®), durvalumab (Imfinzi
®), cemiplimab (Libtayo
®), atezolizumab (Tecentriq
®), cosibelimab and INBRX-105 in several stages of clinical trial in melanoma. Pembrolizumab, nivolumab and nivolumab in combination with iIpilimumab (anti-CTLA-4 inhibitor) have been approved by FDA for treatment of melanoma.
3.1. Pembrolizumab/Lambrolizumab/MK-3475/SCH 900475/Keytruda
This is a humanized monoclonal antibody targeting the PD1 receptor in the lymphocytes. It was developed by Merck and approved for treatment of metastatic melanoma in 2017 [
79].
3.2. Nivolumab/ONO-4538/BMS-936558/MDX1106/Opdivo
This is a human IgG4 anti-PD-1 monoclonal antibody. Nivolumab works as a checkpoint inhibitor that inhibits T-cell activation. [
80]. It was developed by Medarex and Ono Pharmaceutical, and is marketed by Bristol-Myers Squibb (BMS) and Ono. Nivolumab was approved by the FDA for melanoma in 2014 [
81,
82].
3.3. Avelumab/MSB0010718C/Bavencio
This is a humanized monoclonal antibody developed by Merck and Pfizer that targets the PD-L1. It has been approved by FDA for treatment Merkel-cell carcinoma, an aggressive type of skin cancer [
83]. It blocks the PD-1/PD-L1 receptor/ligand complex formation leading to suppression of CD8+ T cells action [
84]. There is a current clinical trial (NCT01772004) investigating the safety, tolerability, pharmacokinetics and clinical activity of avelumab in melanoma [
85].
3.4. Durvalumab/MEDI4736/Imfinzi
This is monoclonal antibody that blocks the interaction of PD-L1 with the PD-1 and CD80 (B7.1) molecules developed by Medimmune/AstraZeneca [
86,
87]. A phase I clinical trial (NCT02586987) is ongoing evaluating the safety and efficacy of selumetinib (AZD6244 Hyd-sulfate) in combination with durvalumab (MEDI4736) along with tremelimumab in patients with advanced solid tumours, including melanoma [
88].
3.5. Atezolizumab/MPDL3280A/Tecentriq
This is a fully humanized engineered monoclonal antibody of IgG1 isotype against PD-L1 developed by Genentech [
89,
90]. There is an active ongoing phase II trial (NCT02303951) which includes the combination of vemurafenib, cobimetinib and atezolizumab in stage III/IV advanced melanoma patients [
91]. Another phase III study (NCT02908672) compares the efficacy of atezolizumab in combination with cobimetinib and vemurafenib versus placebo control plus cobimetinib and vemurafenib in unresectable and advanced melanoma patients with BRAFV600 mutation [
92].
3.6. Spartalizumab/PDR001
Spartalizumab (PDR001) is a humanized monoclonal antibody against the negative immuno-regulatory human cell surface receptor programmed death-1 (PD-1, PCD-1) was developed by Novartis. This suppresses T-cell activation as it binds to PD-1 on activated T-cells and inhibits the interaction with its ligands, (PD-L1, PD-1L1) and (PD-L2, PD-1L2) [
93]. A phase I/Ib (NCT03891953) study evaluating the efficacy of spartalizumab in combination with DKY709 (immunomodulatory agent) in patients with advances solid tumors including melanoma is ongoing [
94]. A phase II PLATforM (NCT03484923) study evaluating the efficacy and safety of spartalizumab in combinations with LAG525 (monoclonal antibody targeting LAG-3), capmatinib (MET inhibitor), canakinumab (monoclonal antibody targeting IL-1β) and ribociclib (CDK4/6 inhibitor) is ongoing in previously treated unresectable or metastatic melanoma [
95]. A phase III COMBI-i study (NCT02967692) comparing the combination of spartalizumab, dabrafenib and trametinib versus dabrafenib and trametinib in previously untreated patients with unresectable or metastatic BRAFV600 mutant melanoma is initiated [
96].
4. Anti-CTLA-4
In addition to PD-1, another immune checkpoint inhibitor, cytotoxic T-lymphocyte antigen 4 (CTLA-4), is important in melanoma. It is found on the surface of regulatory T cells (Treg) and activated T cells [
97]. CTLA-4 competes with CD28, another receptor expressed on the surface of T cells, to interact with its two ligands CD80 and CD86, collectively known as the B7 ligands. When CTLA-4 binds with the B7 ligands, commonly found on antigen presenting cells (APC), it results in an immunosuppressive response, which is the inhibition of T cell activation via transendocytosis of CD80 and CD86 from their surfaces [
98,
99]. Typically, T cell activation requires co-stimulation from the CD28-B7 ligand interaction and the TCR-MHC interaction [
100]. However, CTLA-4 has a stronger affinity for the B7 ligands, making it a good immune checkpoint inhibitor that keeps the immune response from turning into an autoimmune one [
97]. CTLA-4 is expressed on tumor cells, infiltrating Tregs, and exhausted, activated T cells [
101]. Tumor cells, therefore, take advantage of this natural immunosuppressive system in order to prevent an immune response against them. This provides a therapeutic approach which involves anti-CTLA-4 therapy. There are currently three main anti-CTLA-4 antibodies under preclinical and clinical trials for the treatment of melanoma: Tremelimumab, Ipilimumab (Yervoy), and BCD-145.
4.1. Tremelimumab/Ticilimumab/CP 675.206
This human monoclonal antibody against CTLA-4 was developed by AstraZeneca [
102]. A phase I active, clinical trial (NCT02141542) is -evaluating tremelimumab in combination with MEDI3617 (human anti-angiopoietin 2 monoclonal antibody) for unresectable Stage III/IV melanoma patients [
103]. Another phase I active, clinical trial (NCT01103635) is examining tremelimumab in combination with CP-870,893 CD40 agonist monoclonal antibody) for metastatic melanoma [
104].
4.2. Ipilimumab/MDX010/BMS-734016
This human monoclonal antibody against CTLA-4 was developed by YERVOY Medarex/BMS. It was approved by the FDA in 2011 for the treatment of unresectable or metastatic melanoma [
105]. There are current, active clinical trials devoted to assess the efficacy of ipilimumab in combination with other immunotherapies or targeted therapies for metastatic melanoma. A phase I clinical trial (NCT02115243) that assessed ipilimumab as a neoadjuvant followed by melphalan (chemotherapeutic) via isolated limb perfusion in patients with unresectable in-transit extremity melanoma is completed [
106]. A phase Ib clinical trial (NCT02117362) evaluating ipilimumab in combination with GR-MD-02 (galnectin inhibitor) in metastatic melanoma patients has been completed [
107]. A phase II clinical trial (NCT03153085) examining ipilimumab in combination with TBI-1401(HF10) in Japanese patients with Stage IIIb, IIIc, IV unresectable or metastatic malignant melanoma has been completed [
108]. A phase II clinical trial (NCT01970527) looking at SBRT followed by Ipilimumab in patients with stage IV and recurrent melanoma has been completed [
109].
4.3. BCD-145
This human monoclonal antibody against CTLA-4 is developed by BIOCAD [
110]. A completed phase I clinical trial (NCT03472027) studied the efficacy of BCD-145 in unresectable/metastatic melanoma [
111]. The combination of anti-PD-1/PD-L1 and anti-CTLA-4 are also being tested in the clinic for stage III/IV melanoma patients. A phase I clinical trial (NCT02935790) evaluating ipilimumab and nivolumab in combination with ACY-241 (selective HDAC inhibitor) is completed [
112]. Current clinical trials, outcomes and adverse events investigating the efficacy of anti-CTLA-4, anti-PD-1/PD-L1 therapies and their combinations used to treat metastatic melanoma patients are listed in [
113,
114,
115,
116,
117,
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128,
129,
130,
131,
132,
133,
134,
135,
136,
137,
138,
139,
140,
141,
142,
143,
144,
145,
146,
147,
148,
149,
150,
151,
152,
153,
154,
155,
156,
157,
158,
159,
160].
This entry is adapted from the peer-reviewed paper 10.3390/cancers12020482