Abstract: Diabetes mellitus (DM) is a common endocrine disease characterized by a state of
hyperglycemia (higher level of glucose in the blood than usual). DM and its complications can lead
to diabetic foot ulcer (DFU). DFU is associated with impaired wound healing, due to inappropriate
cellular and cytokines response, infection, poor vascularization, and neuropathy. Effective therapeutic
strategies for the management of impaired wound could be attained through a better insight of
molecular mechanism and pathophysiology of diabetic wound healing. Nanotherapeutics-based
agents engineered within 1–100 nm levels, which include nanoparticles and nanoscaffolds, are recent
promising treatment strategies for accelerating diabetic wound healing. Nanoparticles are smaller in
size and have high surface area to volume ratio that increases the likelihood of biological interaction
and penetration at wound site. They are ideal for topical delivery of drugs in a sustained manner,
eliciting cell-to-cell interactions, cell proliferation, vascularization, cell signaling, and elaboration of biomolecules necessary for effective wound healing. Furthermore, nanoparticles have the ability to
deliver one or more therapeutic drug molecules, such as growth factors, nucleic acids, antibiotics,
and antioxidants, which can be released in a sustained manner within the target tissue. This review
focuses on recent approaches in the development of nanoparticle-based therapeutics for enhancing
diabetic wound healing.
- nanoparticle
- drug delivery system
- diabetes mellitus
- wound healing
- diabetic foot ulcer
- pathophysiology
- Introduction
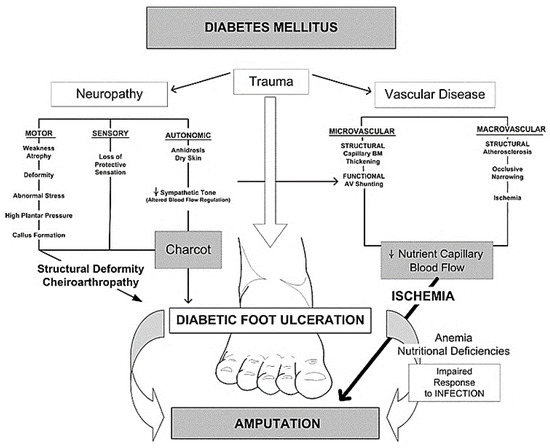
Pathophysiology of Diabetic Foot Ulcer (DFU)
Neuropathy
Peripheral Arterial Disease (PAD)
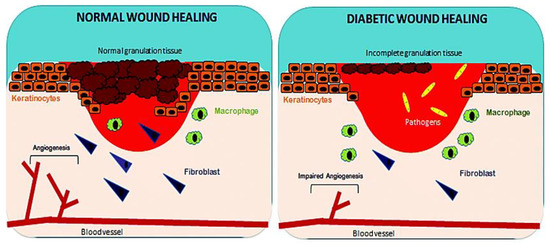
Normal and Diabetic Wound Healing
Therapeutic Modalities for Diabetic Foot Ulcers
Nanotechnology Based Drug Delivery System
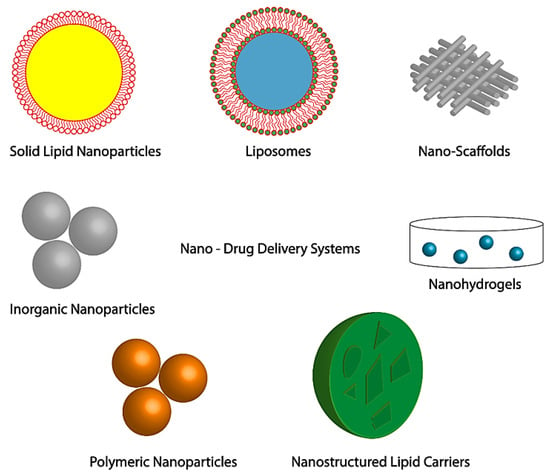
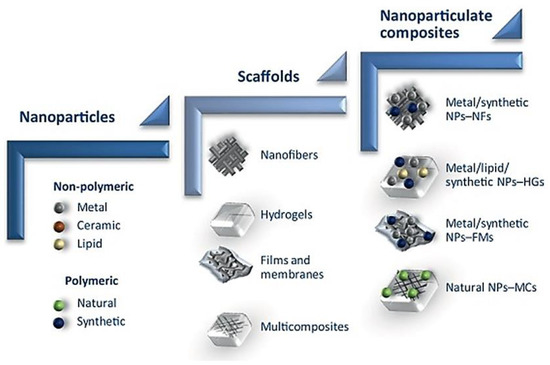
Nanoparticle Delivery of Therapeutic Drugs for Diabetic Wound Healing
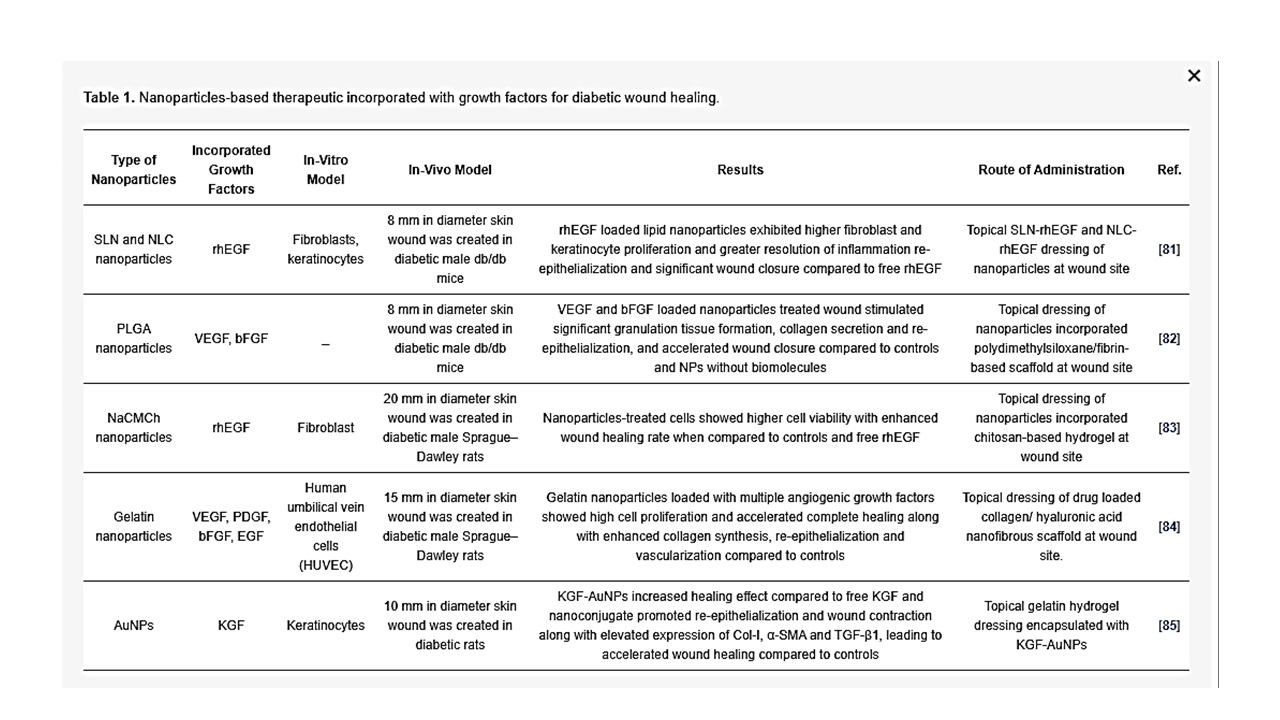
Growth Factors
Nucleic Acid
- (a)
-
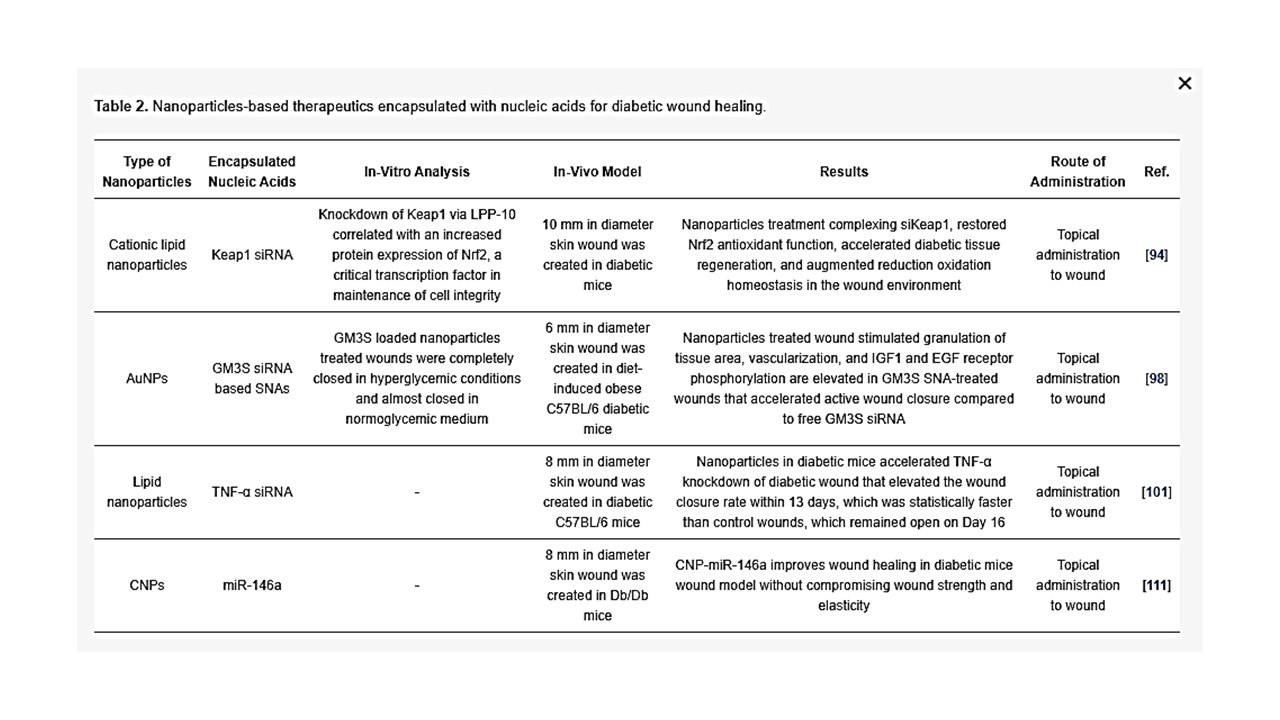
Ganglioside GM3 siRNA: Ganglioside GM3 is a monosialodihexosylganglioside produced by the enzyme GM3 synthase (GM3S). GM3S is key intermediary of insulin resistance which has proven to be highly expressed in human diabetic foot skin, diabetes stimulated obese mouse, hyperglycemic mouse, and mouse keratinocytes exposed to high glucose [96,97]. Randeria et al. showed that knockdown of GM3S expression in diabetic mice by AuNPs conjugated with GMS3 siRNA-based spherical nucleic acids (SNAs) reverse impaired wound healing in diabetic mice with no obvious toxicity [98].
- (b)
-
TNF-α siRNA: TNF-α is an inflammatory cytokine and it is required in limited amounts to accelerate wound healing as TNF-α is required for fibroblast proliferation, migration and wound remodeling. However, in the case of diabetic wound, uncontrolled production of TNF-α blocks the normal process of wound healing by increasing cell apoptosis, ROS and matrix degradation [99,100]. Kasiewicz et al. fabricated lipid nanoparticles encapsulated with specific TNF-α siRNA to accelerate wound healing in diabetic mice [101]. The same investigators demonstrated that topical application of lipid nanoparticles loaded with TNF-α siRNA in the diabetic wound of mice downregulated TNF-α expression by 40–50% with closure of wound significantly faster than control wound.
- (c)
-
Keap1 (Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1) siRNA: In the absence of oxidative stress, the nuclear factor erythroid 2–related factor 2 (Nrf2) binds to Keap1 (in the cytoplasm, which subsequently lead to Nrf2 degradation by ubiquitination). However, in the presence oxidative stress, keap1 is covalently modified in some region that prevents degradation of Nrf2. Following which, Nrf2 enters the nucleus by dissociating from the repressor site of Keap 1 and binds to the antioxidant response element (ARE) in the promotor region of a wide variety of genes responsible for preventing oxidative stress and protein instability, as well as proteasome integrity [102]. ARE is situated in the promoter area of genetic materials that encode many antioxidant and phase II detoxifying enzymes. These enzymes are essential for cellular protection by increasing the elimination of cytotoxic electrophiles and ROS [103]. Chronic hyperglycemia in diabetes causes imbalance of ROS and over production of Keap1, leading to degradation of Nrf2, which regulates diabetic oxidative stress [104,105]. Rabbani et al. has developed a liposome and protein hybrid nanoparticulate delivery system loaded with siRNA specific to Keap1, which can accelerate diabetic wound with severe oxidative stress [94].
- (d)
-
miR-146a: The hyperglycaemic state also activates redox-sensitive transcription factors, mainly NFkB, which leads to over production of pro-inflammatory cytokines such as IL-6 and IL-8 that delay wound healing by extending the inflammation period [106,107]. Cerium oxide nanoparticles (CNP) can act as a therapeutic agent for oxidative stress as CNP has an ability to scavenge free radicals [108,109]. The initial inflammatory response to injury is essential to activate normal wound healing while sustained inflammatory response impairs wound healing associated with diabetic wounds [110]. Zgheib et al. has designed microRNA (miR-146a) loaded CNPs for diabetic wound healing [111]. miR-146a has been reported to negatively regulate the production of pro-inflammatory cytokines, implying that miR-146a can act as a molecular brake in the inflammatory response [112,113]. miR-146a suppresses interlukin-1 receptor associated kinase 1 (IRAK1) and tumor necrosis factor receptor associated kinase 6 (TRAF6), which induces overexpression of IL-6 and IL-8 [114,115]. Down regulation of miR-146a, which influences the upregulation of its target gene IRAK1 and TRAF6, has been observed in diabetic wounds [116]. CNP-miR-146a has been reported to be effective for diabetic wound healings [111]. The use of nanoparticles as a delivery system for siRNA (as summarized in Table 2) may be able to overcome the boundaries of existing methods of free siRNA delivery at wound site because of the capability for encapsulation, controlled release, specific targeting, stability, and bioavailability.
-
-
Table 2. Nanoparticles-based therapeutics encapsulated with nucleic acids for diabetic wound healing.
-
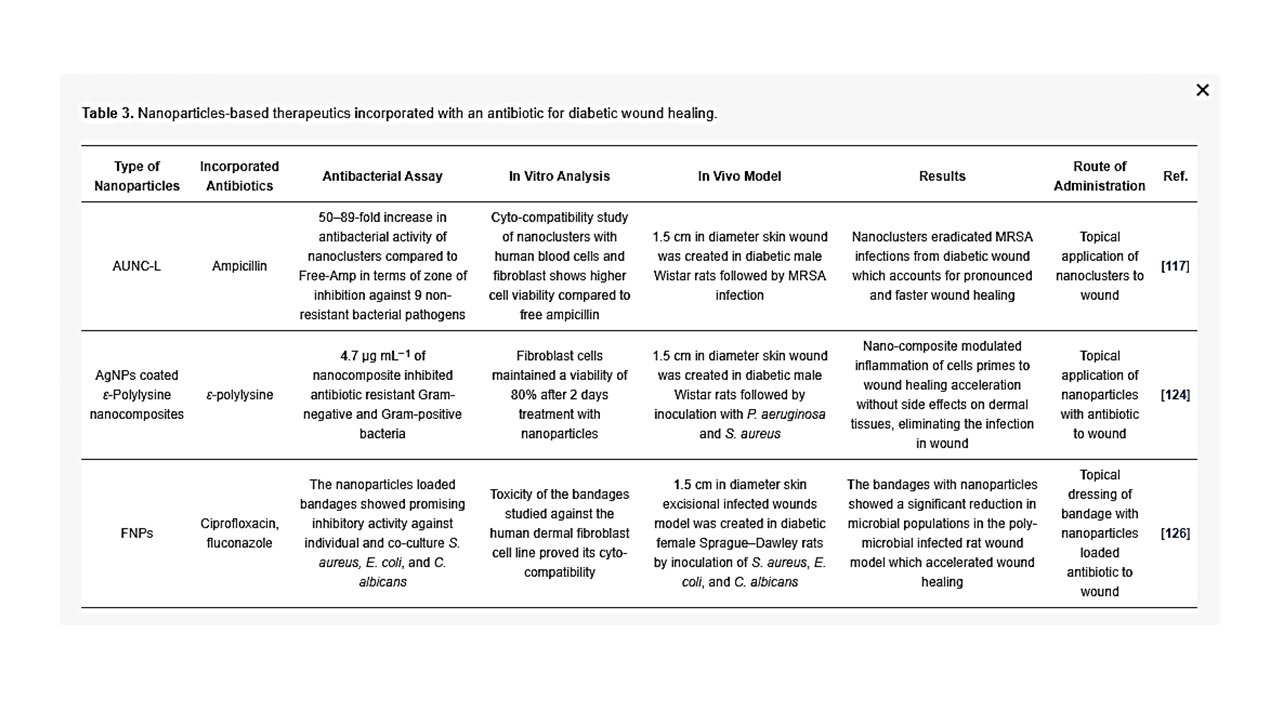
Antibiotics
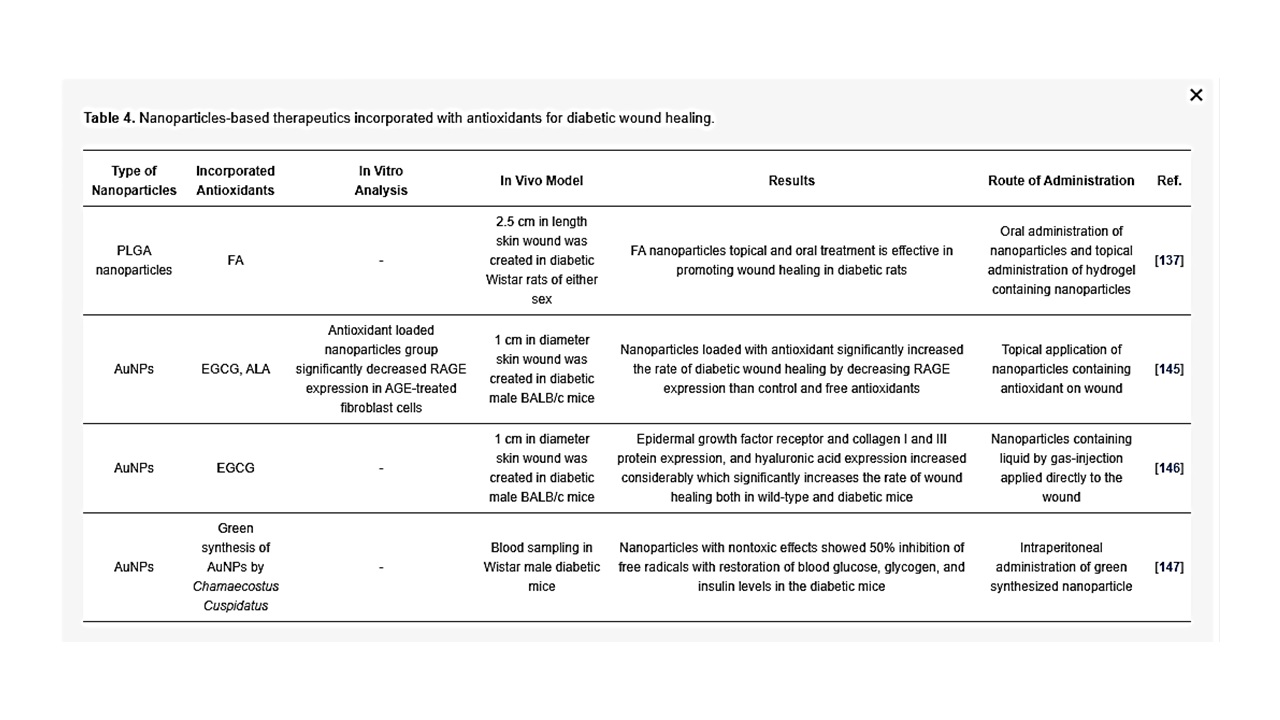
Antioxidants
Regulatory Pathway for Nanomaterial
Clinical Status of Nanomedicine
Future Perspective
Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Kasiewicz, L.N.; Whitehead, K.A. Recent advances in biomaterials for the treatment of diabetic foot ulcers. Biomater. Sci. 2017, 5, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer-A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. 2015, 9, 192–199. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 9th Edition 2019, Global Estimates for the Prevalence of Diabetes for 2019, 2030 and 2045. Available online: http://www.diabetesatlas.org/ (accessed on 30 May 2020).
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. New Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Forlee, M. What is the diabetic foot? The rising prevalence of diabetes worldwide will mean an increasing prevalence of complications such as those of the extremities. Available online: http://www.cmej.org.za/index.php/cmej/article/view/1770 (accessed on 23 June 2020).
- Wu, S.C.; Driver, V.R.; Wrobel, J.S.; Armstrong, D.G. Foot ulcers in the diabetic patient, prevention and treatment. Vasc. Health Risk Manag. 2007, 3, 65–76. [Google Scholar]
- Frykberg, R.G.; Zgonis, T.; Armstrong, D.G.; Driver, V.R.; Giurini, J.M.; Kravitz, S.R.; Landsman, A.S.; Lavery, L.A.; Moore, J.C.; Schuberth, J.M.; et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J. Foot Ankle. Surg. 2006, 45, S1–S66. [Google Scholar] [CrossRef]
- Bowering, C.K. Diabetic foot ulcers. Pathophysiology, assessment, and therapy. Can. Fam. Phys. 2001, 47, 1007–1016. [Google Scholar]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Clayton, W.; Elasy, T.A. A Review of the Pathophysiology, Classification, and Treatment of Foot Ulcers in Diabetic Patients. Clin. Diabetes 2009, 27, 52–58. [Google Scholar] [CrossRef]
- Simmons, Z.; Feldman, E.L. Update on diabetic neuropathy. Curr. Opin. Neurol. 2002, 15, 595–603. [Google Scholar] [CrossRef]
- Juster-Switlyk, K.; Smith, A.G. Updates in diabetic peripheral neuropathy. F1000Research 2016, 5, 738. [Google Scholar] [CrossRef]
- Avogaro, A.; Albiero, M.; Menegazzo, L.; de Kreutzenberg, S.; Fadini, G.P. Endothelial dysfunction in diabetes: The role of reparatory mechanisms. Diabetes Care 2011, 34, S285–S290. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Jan, A.; Wajid, M.A.; Tariq, S. Management of Chronic Non-healing Wounds by Hirudotherapy. World J. Plast. Surg. 2017, 6, 9–17. [Google Scholar] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.F. The role of mast cells in wound healing. Int. Wound J. 2010, 7, 55–61. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef]
- Shah, J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Monaco, J.L.; Lawrence, W.T. Acute wound healing an overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Cho, H.; Blatchley, M.R.; Duh, E.J.; Gerecht, S. Acellular and cellular approaches to improve diabetic wound healing. Adv. Drug Deliv. Rev. 2019, 146, 267–288. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. Biomed. Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral. Health 2015, 7, 75–80. [Google Scholar]
- Nguyen, T.; Mobashery, S.; Chang, M. Roles of Matrix Metalloproteinases in Cutaneous Wound Healing; IntechOpen Ltd.: London, UK, 2016; pp. 37–71. [Google Scholar]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef]
- Gottrup, F.; Apelqvist, J. Present and new techniques and devices in the treatment of DFU: A critical review of evidence. Diabetes Metab. Res. Rev. 2012, 28, 64–71. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Xu, W.; Yuan, F.; Chu, D.; Ding, Y.; Chen, B.; Jafari, M.; Yuan, Y.; Chen, P. A novel peptide for efficient siRNA delivery in vitro and therapeutics in vivo. Acta Biomater. 2015, 21, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Mordorski, B.; Rosen, J.; Friedman, A. Nanotechnology as an innovative approach for accelerating wound healing in diabetes. Diabetes Manag. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Jackson, J.E.; Kopecki, Z.; Cowin, A.J. Nanotechnological Advances in Cutaneous Medicine. J. Nanomater. 2013, 8, 808234. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Korrapati, P.S.; Karthikeyan, K.; Satish, A.; Krishnaswamy, V.R.; Venugopal, J.R.; Ramakrishna, S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Wang, Y. Drug delivery systems for wound healing. Curr. Pharm. Biotechnol. 2015, 16, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Berthet, M.; Gauthier, Y.; Lacroix, C.; Verrier, B.; Monge, C. Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017, 35, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.T.; Kirby, G.; Jayakumar, R.; Liang, X.J.; Tan, A. Accelerated Wound Healing Using Nanoparticles. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ezhilarasu, H.; Ramalingam, R.; Dhand, C.; Lakshminarayanan, R.; Sadiq, A.; Gandhimathi, C.; Ramakrishna, S.; Bay, B.H.; Venugopal, J.R.; Srinivasan, D.K. Biocompatible Aloe vera and Tetracycline Hydrochloride Loaded Hybrid Nanofibrous Scaffolds for Skin Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5174. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ezhilarasu, H.; Prasannan, P.; Ong, S.T.; Subramanian, S.; Kamruddin, M.; Lakshminarayanan, R.; Ramakrishna, S.; et al. Poly-ε-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study. Nanomaterials 2019, 9, 462. [Google Scholar] [CrossRef]
- Shan, X.; Liu, C.; Li, F.; Ouyang, C.; Gao, Q.; Zheng, K. Nanoparticles vs. nanofibers: A comparison of two drug delivery systems on assessing drug release performance in vitro. Des. Monomers Polym. 2015, 18, 678–689. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Sadiq, A.; Ratheesh, G.; Sridhar, S.; Ramakrishna, S.; Ab Rahim, M.H.; Yusoff, M.M.; Jose, R.; Reddy, V.J. Functionalized core/shell nanofibers for the differentiation of mesenchymal stem cells for vascular tissue engineering. Nanomedicine 2019, 14, 201–214. [Google Scholar] [CrossRef]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Thamphiwatana, S.; Lu, D.; Zhang, L. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxid. Med. Cell Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Alarcon, E.C.; Griffith, M.; Udekwu, K.I. Silver Nanoparticle Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Akturk, O.; Kismet, K.; Yasti, A.C.; Kuru, S.; Duymus, M.E.; Kaya, F.; Caydere, M.; Hucumenoglu, S.; Keskin, D. Collagen/gold nanoparticle nanocomposites: A potential skin wound healing biomaterial. J. Biomater. Appl. 2016, 31, 283–301. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- El-Gharbawy, R.M.; Emara, A.M.; Abu-Risha, S.E. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed Pharm. 2016, 84, 810–820. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, X.; Xu, Z.; Yi, C. ZnO-based nanocarriers for drug delivery application: From passive to smart strategies. Int. J. Pharm. 2017, 534, 190–194. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sheldon, B.; Webster, T. Nanophase ceramics for improved drug delivery: Current opportunities and challenges. Am. Ceram. Soc. Bull. 2010, 89, 24–32. [Google Scholar]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, J.H.; Hong, Y.S. Development of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Blažević, F.; Milekić, T.; Romić, M.D.; Juretić, M.; Pepić, I.; Filipović-Grčić, J.; Lovrić, J.; Hafner, A. Nanoparticle-mediated interplay of chitosan and melatonin for improved wound epithelialisation. Carbohydr. Polym. 2016, 146, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Stone, W.L.; Varacallo, M. Physiology, Growth Factor. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Ulubayram, K.; Cakar, A.N.; Korkusuz, P.; Ertan, C.; Hasirci, N. EGF containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Mast, B.A.; Schultz, G.S. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996, 4, 411–420. [Google Scholar] [CrossRef]
- Mark Saltzman, W.; Baldwin, S.P. Materials for protein delivery in tissue engineering. Adv. Drug Deliv. Rev. 1998, 33, 71–86. [Google Scholar] [PubMed]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yu, D.; Wang, P.; Xu, J.; Li, D.; Ding, M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Lopes, A.; Koussoroplis, S.; Payen, V.; Moia, C.; Zhu, H.; Sonveaux, P.; Carmeliet, P.; des Rieux, A.; Vandermeulen, G.; et al. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine 2015, 11, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Briganti, E.; Errico, C.; Lisella, A.; Sanguinetti, E.; Chiellini, F.; Soldani, G. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013, 9, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Hajimiri, M.; Shahverdi, S.; Esfandiari, M.A.; Larijani, B.; Atyabi, F.; Rajabiani, A.; Dehpour, A.R.; Amini, M.; Dinarvand, R. Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev. Ind. Pharm. 2016, 42, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Li, S.; Tang, Q.; Xu, H.; Huang, Q.; Wen, Z.; Liu, Y.; Peng, C. Improved stability of KGF by conjugation with gold nanoparticles for diabetic wound therapy. Nanomedicine 2019, 14, 2909–2923. [Google Scholar] [CrossRef]
- Uchi, H.; Igarashi, A.; Urabe, K.; Koga, T.; Nakayama, J.; Kawamori, R.; Tamaki, K.; Hirakata, H.; Ohura, T.; Furue, M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur. J. Dermatol. 2009, 19, 461–468. [Google Scholar] [CrossRef]
- Hanft, J.R.; Pollak, R.A.; Barbul, A.; van Gils, C.; Kwon, P.S.; Gray, S.M.; Lynch, C.J.; Semba, C.P.; Breen, T.J. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J. Wound Care 2008, 17, 30–32, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, L.; Gao, C. Design of gene-activated matrix for the repair of skin and cartilage. Polym. J. 2014, 46, 476–482. [Google Scholar] [CrossRef]
- Kwon, M.J.; An, S.; Choi, S.; Nam, K.; Jung, H.S.; Yoon, C.S.; Ko, J.H.; Jun, H.J.; Kim, T.K.; Jung, S.J.; et al. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J. Gene Med. 2012, 14, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Jafari, S.; Khosroushahi, A.Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Kasiewicz, L.N.; Whitehead, K.A. Silencing TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1 in an in vitro co-culture model of diabetic foot ulcers. Acta Biomater. 2016, 32, 120–128. [Google Scholar] [CrossRef]
- Jozic, I.; Daunert, S.; Tomic-Canic, M.; Pastar, I. Nanoparticles for Fidgety Cell Movement and Enhanced Wound Healing. J. Invest. Dermatol. 2015, 135, 2151–2153. [Google Scholar] [CrossRef]
- Rabbani, P.S.; Zhou, A.; Borab, Z.M.; Frezzo, J.A.; Srivastava, N.; More, H.T.; Rifkin, W.J.; David, J.A.; Berens, S.J.; Chen, R.; et al. Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials 2017, 132, 1–15. [Google Scholar] [CrossRef]
- Zhou, J.; Shum, K.T.; Burnett, J.C.; Rossi, J.J. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals 2013, 6, 85–107. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lee, S.; Wilson, H.; Seeger, M.; Iordanov, H.; Gatla, N.; Whittington, A.; Bach, D.; Lu, J.Y.; Paller, A.S. Ganglioside GM3 depletion reverses impaired wound healing in diabetic mice by activating IGF-1 and insulin receptors. J. Invest. Dermatol. 2014, 134, 1446–1455. [Google Scholar] [CrossRef]
- Tagami, S.; Inokuchi Ji, J.; Kabayama, K.; Yoshimura, H.; Kitamura, F.; Uemura, S.; Ogawa, C.; Ishii, A.; Saito, M.; Ohtsuka, Y.; et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 2002, 277, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Randeria, P.S.; Seeger, M.A.; Wang, X.Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bal, H.S.; Desta, T.; Behl, Y.; Graves, D.T. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am. J. Pathol. 2006, 168, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Born, K.; Barker, J.H.; Marzi, I. In Vivo Effect of Tumor Necrosis Factor Alpha on Wound Angiogenesis and Epithelialization. Eur. J. Trauma 2003, 29, 208–219. [Google Scholar] [CrossRef]
- Kasiewicz, L.N.; Whitehead, K.A. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng. Transl. Med. 2019, 4, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2017, 161, 1–13. [Google Scholar] [CrossRef]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef]
- auf dem Keller, U.; Kümin, A.; Braun, S.; Werner, S. Reactive oxygen species and their detoxification in healing skin wounds. J. Investig. Dermatol. Symp. Proc. 2006, 11, 106–111. [Google Scholar] [CrossRef]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; auf dem Keller, U.; Born-Berclaz, C.; Chan, K.; Kan, Y.W.; Werner, S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef]
- Zgheib, C.; Hodges, M.M.; Hu, J.; Liechty, K.W.; Xu, J. Long non-coding RNA Lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages. PLoS ONE 2017, 12, e0177453. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos. Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef] [PubMed]
- Garash, R.; Bajpai, A.; Marcinkiewicz, B.M.; Spiller, K.L. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp. Biol. Med. 2016, 241, 1054–1063. [Google Scholar] [CrossRef]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of Cerium Oxide Nanoparticles Conjugated with MicroRNA-146a to Correct the Diabetic Wound Healing Impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Dunne, N.; McCarthy, H.O. MicroRNA as Therapeutic Targets for Chronic Wound Healing. Mol. Ther. Nucleic Acids 2017, 8, 46–55. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, L.; Luo, Q.; Wu, M.; Chen, Y.; Shi, X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des. Devel. Ther. 2018, 12, 171–177. [Google Scholar] [CrossRef]
- Lo, W.Y.; Peng, C.T.; Wang, H.J. MicroRNA-146a-5p Mediates High Glucose-Induced Endothelial Inflammation via Targeting Interleukin-1 Receptor-Associated Kinase 1 Expression. Front. Physiol. 2017, 8, 551. [Google Scholar] [CrossRef]
- Xu, J.; Wu, W.; Zhang, L.; Dorset-Martin, W.; Morris, M.W.; Mitchell, M.E.; Liechty, K.W. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: Correction with mesenchymal stem cell treatment. Diabetes 2012, 61, 2906–2912. [Google Scholar] [CrossRef]
- Kalita, S.; Kandimalla, R.; Bhowal, A.C.; Kotoky, J.; Kundu, S. Functionalization of β-lactam antibiotic on lysozyme capped gold nanoclusters retrogress MRSA and its persisters following awakening. Sci. Rep. 2018, 8, 5778. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Thambi, T.; Yang, Y.H.; Bang, S.; Kim, B.S.; Pyun, D.G.; Lee, D.S. AgNP and rhEGF-incorporating synergistic polyurethane foam as a dressing material for scar-free healing of diabetic wounds. RSC Adv. 2017, 7, 13714–13725. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; S, M.; Padwad, Y.S.; Yadav, S.K. Cytocompatible Anti-microbial Dressings of Syzygium cumini Cellulose Nanocrystals Decorated with Silver Nanoparticles Accelerate Acute and Diabetic Wound Healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef]
- Salouti, M.; Ahangari, A. Nanoparticle based drug delivery systems for treatment of infectious diseases. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; IntechOpen Ltd.: London, UK, 2014; pp. 155–194. [Google Scholar]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Dai, X.; Guo, Q.; Zhao, Y.; Zhang, P.; Zhang, T.; Zhang, X.; Li, C. Functional Silver Nanoparticle as a Benign Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 25798–25807. [Google Scholar] [CrossRef]
- Brogden, K.A.; Guthmiller, J.M.; Taylor, C.E. Human polymicrobial infections. Lancet 2005, 365, 253–255. [Google Scholar] [CrossRef]
- Thattaruparambil Raveendran, N.; Mohandas, A.; Ramachandran Menon, R.; Somasekharan Menon, A.; Biswas, R.; Jayakumar, R. Ciprofloxacin- and Fluconazole-Containing Fibrin-Nanoparticle-Incorporated Chitosan Bandages for the Treatment of Polymicrobial Wound Infections. ACS Appl. Bio. Mater. 2019, 2, 243–254. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef]
- He, J.; Liang, Y.; Shi, M.; Guo, B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly (ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Tauler Riera, P. Redox Status. In Encyclopedia of Exercise Medicine in Health and Disease; Mooren, F.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 751–753. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Liemburg-Apers, D.C.; Willems, P.H.; Koopman, W.J.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative stress and human hypertension: Vascular mechanisms, biomarkers, and novel therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt Ann, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Shao, W.; Gao, J.; Ling, D. Promoting Angiogenesis in Oxidative Diabetic Wound Microenvironment Using a Nanozyme-Reinforced Self-Protecting Hydrogel. ACS Cent. Sci. 2019, 5, 477–485. [Google Scholar] [CrossRef]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef]
- Huijberts, M.S.P.; Schaper, N.C.; Schalkwijk, C.G. Advanced glycation end products and diabetic foot disease. Diabetes Metab. Res. Rev. 2008, 24, S19–S24. [Google Scholar] [CrossRef]
- Niu, Y.; Xie, T.; Ge, K.; Lin, Y.; Lu, S. Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am. J. Dermatopathol. 2008, 30, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Peres, G.B.; Schor, N.; Michelacci, Y.M. Impact of high glucose and AGEs on cultured kidney-derived cells. Effects on cell viability, lysosomal enzymes and effectors of cell signaling pathways. Biochimie 2017, 135, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F.; et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef]
- Liang, Y.J.; Jian, J.H.; Liu, Y.C.; Juang, S.J.; Shyu, K.G.; Lai, L.P.; Wang, B.W.; Leu, J.G. Advanced glycation end products-induced apoptosis attenuated by PPARdelta activation and epigallocatechin gallate through NF-kappaB pathway in human embryonic kidney cells and human mesangial cells. Diabetes Metab. Res. Rev. 2010, 26, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, K.W. Protective effect of (-)-epigallocatechin gallate against advanced glycation endproducts-induced injury in neuronal cells. Biol. Pharm. Bull. 2007, 30, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Lateef, H.; Aslam, M.N.; Stevens, M.J.; Varani, J. Pretreatment of diabetic rats with lipoic acid improves healing of subsequently-induced abrasion wounds. Arch. Dermatol. Res. 2005, 297, 75–83. [Google Scholar] [CrossRef]
- Chen, S.A.; Chen, H.M.; Yao, Y.D.; Hung, C.F.; Tu, C.S.; Liang, Y.J. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012, 47, 875–883. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chen, C.-Y.; Chen, P.-J.; Tan, S.-W.; Chen, C.-N.; Chen, H.-M.; Tu, C.-S.; Liang, Y.-J. Gas-injection of gold nanoparticles and anti-oxidants promotes diabetic wound healing. RSC Adv. 2014, 4, 4656–4662. [Google Scholar] [CrossRef]
- Ponnanikajamideen, M.; Rajeshkumar, S.; Vanaja, M.; Annadurai, G. In Vivo Type 2 Diabetes and Wound-Healing Effects of Antioxidant Gold Nanoparticles Synthesized Using the Insulin Plant Chamaecostus cuspidatus in Albino Rats. Can. J. Diabetes 2019, 43, 82–89. [Google Scholar] [CrossRef]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Administration, D. Guidance for industry considering whether an FDA-regulated product involves the application of nanotechnology. Biotechnol. Law Rep. 2011, 30, 613–616. [Google Scholar]
- Tyner, K.M.; Zou, P.; Yang, X.; Zhang, H.; Cruz, C.N.; Lee, S.L. Product quality for nanomaterials: Current U.S. experience and perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupančič, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Juillerat-Jeanneret, L.; Dusinska, M.; Fjellsbo, L.M.; Collins, A.R.; Handy, R.D.; Riediker, M. Biological impact assessment of nanomaterial used in nanomedicine. Introduction to the NanoTEST project. Nanotoxicology 2015, 9, 5–12. [Google Scholar] [CrossRef]
- Anderson, J.M. Biocompatibility. Polym. Sci. A Compr. Ref. 10 Vol. Set 2012, 9, 363–383. [Google Scholar]
- Hussain, S.M.; Warheit, D.B.; Ng, S.P.; Comfort, K.K.; Grabinski, C.M.; Braydich-Stolle, L.K. At the crossroads of nanotoxicology in vitro: Past achievements and current challenges. Toxicol. Sci. 2015, 147, 5–16. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Keck, C.M.; Müller, R.H. Nanotoxicological classification system (NCS)—A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur. J. Pharm. Biopharm. 2013, 84, 445–448. [Google Scholar] [CrossRef]
- Han, H. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar]
- Layliev, J.; Wilson, S.; Warren, S.M.; Saadeh, P.B. Improving Wound Healing with Topical Gene Therapy. Adv. Wound Care 2012, 1, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Karponis, D.; Mosahebi, A.; Seifalian, A.M. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front. Biosci. 2018, 23, 1038–1059. [Google Scholar]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tsen, F.; Sahu, D.; Bhatia, A.; Chen, M.; Multhoff, G.; Woodley, D.T. Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: Intentionally or unintentionally. Int. Rev. Cell Mol. Biol. 2013, 303, 203–235. [Google Scholar]
- Khvalevsky, E.Z.; Gabai, R.; Rachmut, I.H.; Horwitz, E.; Brunschwig, Z.; Orbach, A.; Shemi, A.; Golan, T.; Domb, A.J.; Yavin, E.; et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20723–20728. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
This entry is adapted from the peer-reviewed paper 10.3390/nano10061234