Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Delaying the nodule senescence of legume crops can prolong the time of nitrogen fixation and attenuate the lack of fertilizer in the later stage of legume crop cultivation, resulting in improved crop yield and reduced usage of nitrogen fertilizer.
- legume-rhizobium symbiosis
- nodule senescence
- N-fixation activity
- cysteine proteases
- abiotic factors
1. Introduction
Nitrogen is one of the most important limiting factors for the growth and development of plants in nature. At present, the high yield of crops largely depends on the use of nitrogen fertilizers. However, large-scale application of nitrogen fertilizers not only increases production costs but also causes environmental pollution. As a biological process between nitrogen-fixing microorganisms and legumes, symbiotic nitrogen fixation (SNF) directly converts atmospheric nitrogen into ammonia through nitrogenase [1]. Therefore, it is the most economical and environmentally-friendly way of achieving nitrogen fixation [2]. Moreover, SNF plays a significant role in enhancing the recovery of grassland ecosystems, promoting the growth of windbreak and sand fixation tree species, accelerating the remediation of heavy metal-contaminated land, improving the composition of soil nutrients, and ameliorating the agricultural weight loss and efficiency [3][4].
The formation of legume-rhizobium symbiosis is the result of mutual recognition between microorganisms and plants, as well as the interaction between signaling molecules [5]. First, flavonoid compounds released by the legume roots can induce rhizobia to produce Nod factors, which have the function as signal molecules and can be recognized by Nod factor receptors of legume plants. Then, the root hairs of legumes curl, deform and form infection threads [6] through which rhizobia penetrate the root tissue [7]. Simultaneously, some cortical cells of the root are stimulated to start dividing to form a primordial nodule [8]. The cells infected by the bacteria derive from root inner cortex cells, which differentiate and then proliferate to form the nodule meristem. The timing of initiation, development, and maturing of nodule organogenesis inside root cells has been well documented [9][10].
Nodules in legumes can be divided into two types depending on the persistence of the nodule meristem as follows: determinate nodules and indeterminate nodules, which have different characteristics in morphology [11]. Indeterminate nodules, such as those in Medicago truncatula, Pisum sativum, Vicia faba, and Trifolium repens, contain five different zones: the active meristem that allows the growth of the nodule (Zone I), the infection zone, where the cells differentiate and are infected by the bacteria (Zone II), the nitrogen fixation zone (Zone III), in which bacteroids fix atmospheric nitrogen, and the senescent zone (Zone IV), where bacteria and plant cells are deconstructed, accompanied by the destruction of symbiosis [12]. Determinate nodules, such as Glycine max and Lotus japonicus, have non-persistent meristems, and all the infected cells are always at the similar stage of development. In both determinate and indeterminate nodules, rhizobia supply nitrogen sources for the growth and development of legume plants by SNF, and in return, legumes provide carbon sources to rhizobia to sustain their growth and reproduction within the nodules [13].
There are three major approaches to increase SNF efficiency: increasing the number of nodules to improve nitrogen fixation efficiency; improving nitrogen-fixing enzyme activity, and delaying nodule senescence to extend the time of nitrogen fixation and increase nitrogen fixation amount. Among them, delaying nodule senescence and prolonging the time of nitrogen fixation are the key measures to ensure nitrogen fixation and adequate supply of nitrogen to legumes in the reproductive period and to solve the problem of late defertilization in legumes, which often occurs in pod stage when the symbiotic nitrogen fixation ability of legume crops is gradually weakened, and meanwhile it is hard to artificially supply extra nitrogen fertilizer to meet the demand at this stage.
2. Characteristics and Research Methods of Legume Nodule Senescence
Generally, the efficiency of nitrogen fixation in legume plants reaches a peak at 4–6 weeks after inoculation with rhizobia, after which the bacteroids differentiate and the nitrogen fixation ability is gradually weakened [14]. Nodule senescence is a complex and programmed process, including the reduction of Leghemoglobin content, structural changes of nodule cells, the decrease of nitrogenase activity, changes in the lifestyle and growth capacity of bacteria, and so on. The morphology, physiological and biochemical changes and the corresponding methods are summarized in Table 1.
During the nodule senescence, in particular, the color of the nitrogen fixation zone is converted from pink to green due to the nitration of the heme group of functional leghemoglobin (LHb) [15]. This nodule color change is caused by nitrogen biomobilization and a rapid decrease in LHb content [16]. Therefore, we can assess the degree of nodule senescence by calculating the ratio of senescent nodules to the total nodules by observing the changes in nodule color, and detect the content of LHb by using the cyanmethemoglobin method, which is a rapid, quantitative method for the determination of LHb in legume nodules [17][18]. Besides, we can also examine the content of LHb at the transcriptional and protein levels by using qPCR and Western blotting analysis.
The most representative characteristic of nodule senescence is the structural changes of the nodule cells, including progressively reduced electron density, the appearance of numerous vesicles in the cytoplasm, increased number of peroxisomes, the formation of complex elongated structures by mitochondria, symbiotic membrane disintegration, damaged cell wall, and lysis of bacteroids [14][19][20]. Generally, paraffin sections of nodules are made to observe the dynamic changes of senescence zone [21], the TdT-mediated dUTP-biotin nick end-labeling (TUNEL) staining assay is used to detect the programmed cell death or apoptotic-like cell death in nodules [22][23], transmission electron microscopy is utilized to observe the morphology of the inner cells, the electron density of the symbiosis, and the integrity of the symbiotic membrane [23], and in situ live/dead staining assay has been found to detect the rhizobia with intact cell membranes or damaged membranes [24][25].
Table 1. The morphology, physiological and biochemical changes of legume nodule senescence and the corresponding detection methods.
Decreased nitrogenase activity, lifestyle and growth capacity of bacteria are also the major features of nodule senescence [26][27]. The decline in nitrogen-fixing enzyme activity is measured using the acetylene reduction assay (ARA), which is a high sensitivity, rapid, low cost, and nondestructive method [28][29]. Rhizobium is an important player in nodule senescence, and the morphological and physiological characteristics of senescent nodules have revealed that half of the rhizobia surviving in a hemibiotrophic-like lifestyle cannot grow [26][27]. Therefore, testing the lifestyle and growth capacity of bacteria is also an efficient method to evaluate nodule senescence.
Legumes can control the formation of symbiosis and the lifespan of nodules through the molecular dialogue between metabolites and hormones [5][30]. Therefore, nodule senescence can be detected by measuring the metabolites and hormone signals that determine the onset of nodule senescence in plants [31]. For example, endogenous ethylene plays a role in the formation and position of nodule primordia [32][33][34].Two ethylene inhibitors silver ions (Ag+) and aminoethoxy-vinylglycine (AVG) can counteract the interfering effect of ethylene, we therefore measured the position of nodule formation in the presence of AVG or Ag + to test whether the endogenous ethylene provided the position information to control the formation of nodule primordia [34].Furthermore, according to known molecular markers associated with nodule senescence, several methods such as laser microdissection, qPCR, and ACC immunolocalization are employed for nodule senescence detection [35].
3. Gene Regulation Related to Legume Nodule Senescence
Delaying nodule senescence and/or prolonging nitrogen fixation time are the key measures to solve the problem of late defertilization of legumes, which is critical for the planting of legumes and agriculture. Nodule senescence is a genetically controlled process, and transcriptomic studies of the model legumes L. japonicus and M. truncatula have identified lots of up- or down-regulated genes in senescent nodules [19][36][37][38]. In soybean, we analyzed the nodule development at five different developmental stages (branching stage, flowering stage, fruiting stage, pod stage and harvest stage), and found many nodule senescence-related genes, including those encoding soybean cysteine proteases, cystatins, cysteine-rich proteins and transcription factors, as well as proteins involved in plant-pathogen interactions [21]. We performed whole-genome surveys of soybean papain-like cysteine protease (PLCP) family genes [39], soybean cystatin family genes [40] and soybean C2H2 zinc finger family genes [41], analyzed their expression profiles during nodule development and senescence, and identified dozens of candidate genes related to nodule senescence. Besides, many genes have been studied to play roles in regulating the nodule senescence of legume plants in recent years. These genes mainly include cysteine proteases, transcription factors, peptides, enzymes, and other functional genes (Figure 1).
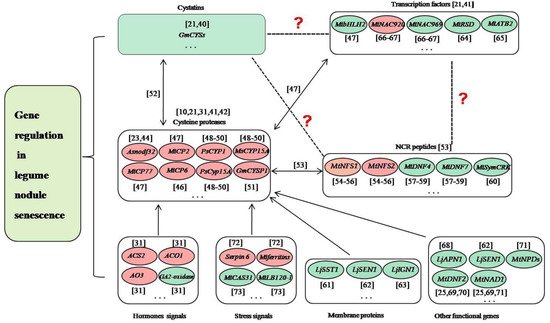
Figure 1. Prediction of the relationships among cysteine proteases, cystatins, NCR proteins, transcription factors and other genes in the regulation of nodule senescence. The dotted lines and/or question marks indicate that the relationship between the two proteins is uncertain. The double arrows indicate that the direct regulatory relationship between the two proteins is proved. The single arrows indicate that the relationship between the two proteins exists. The red ellipses indicate that the genes positively regulate nodule senescence. The green ellipses indicate that the genes negatively regulate nodule senescence. The ellipsis indicates that many genes have not yet been identified [10][21][23][25][35][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69].
During the progress of nodule senescence, the expressions of various hydrolases are up-regulated to trigger large-scale protein degradation, which is a hallmark of nodule senescence, especially for cysteine proteases [42]. Cysteine proteases are a class of proteins widely involved in the senescence and programmed cell death of various tissues and organs of plants [70], and they have been shown to play key roles in legume nodule senescence [10][35]. In Astragalus sinicus, Asnodf32 is a nodule-specific cysteine protease and RNAi interference with the expression of Asnodf32 delays nodule senescence and prolongs nodule lifespan [23][43]. In M. truncatula, Mtcp1-Mtcp6, a conserved cysteine protease subfamily, may be involved in the degradation of symbiont structure [71], and MtCP6 promotes nodule senescence [44]. MtCP77 positively regulates nodule senescence by accelerating plant PCD and reactive oxygen species (ROS) accumulation [45]. In pea, PsCYP1 is expressed at the onset of senescence in the indeterminate nodules, and inhibition of PsCyp15A or MsCYP15A delays the senescence of nodules [46][47][48]. In soybean, GmCYSP1 may participate in nodule development and senescence [49]. Transcriptomic studies have shown that dozens of papain-like cysteine proteases (PLCPs) are associated with nodule senescence [21][72]. We performed the genome-wide survey of PLCPs to detect their expression in root nodule symbiosis, and identified many nodule senescence-related PLCPs [39]. Moreover, cysteine protease can specifically inhibit nodule cysteine-rich (NCR) peptides, which mainly exist in legumes and are closely related to the terminal differentiation of rhizobia [73]. For example, MtNFS1 and MtNFS2 can induce bacterial death and early nodule senescence in rhizobial strain-dependent manner [50][51][52]. MtDNF4 and MtDNF7 are essential for bacterial survival, bacterial differentiation and nitrogen fixation [53][54][55]. MtSymCRK can prevent nodule senescence and avoid defense-like reactions [56]. Cysteine protease can be inhibited by its natural inhibitor cystatin, several of which are differently expressed in symbiosis and play roles in nodule senescence [21][40][72]. Cysteine protease can also be regulated by transcription factors during nodule senescence. For example, MtbHLH2 can repress MtCP77 to negatively regulate nodule senescence [45]. MtNAC920 can up-regulate MtCP2 to play positive roles in nodule senescence [45]. These findings suggested complex relationships may exist among cysteine proteases, cystatins, NCR proteins and transcription factors. In the regulation of the root nodule senescence of legumes, previous studies have proved that cystatins and transcription factors can regulate cysteine proteases, cysteine proteases can inhibit NCR proteins, while whether transcription factors can regulate cystatins and NCR proteins, and whether cystatins can regulate NCR proteins remain unclear (Figure 1).
Nodule senescence is coregulated by legumes and rhizobia, and a series of cellular metabolic processes and the transport of nutrients are involved in this process. In legume plants, except for cysteine proteases, the genes associated with these biological processes mainly are membrane proteins, transcription factors and other functional genes. LjSST1, which is a nodule-specific sulfate transporter that is located on the symbiosome membrane in Lotus nodules, is critical for symbiotic nitrogen fixation [57]. LjSEN1, encoding an integral membrane protein, plays important roles in regulating the nitrogen fixation activity and the differentiation of bacteroids or symbiosomes [58]. LjIGN1 is an ankyrin-repeat membrane protein and is essential for bacteroid differentiation and functioning [59]. Many soybean C2H2 transcription factors may play key roles in nodule senescence [41]. MtRSD, which encodes a nodule-specific C2H2 transcription factor, can repress the transcription of VAMP721a and promote symbiosome development in M.truncatula [60]. A bZIP transcription factor MtATB2, which can regulate carbon metabolism and metabolite partitioning, is regulated by sucrose and enhanced during nodule senescence [61]. Two NAC transcription factors MtNAC969 and MtNAC920 can be induced by root abiotic stress and have been shown to play roles in nodule senescence [62][63]. bHLH2 transcription factor MtbHLH2 plays significant roles in plant PCD, ROS accumulation, and nodule senescence [45]. LjAPN1 is a nepenthesin-type aspartic peptidase, and plays critical roles in the persistence of nitrogen-fixing symbiosis functioning in a rhizobial strain-dependent manner [64]. MtNAD1 and MtDNF2 are essential for the maintenance of rhizobial endosymbiosis and the control of plant defense during the symbiotic colonization [25][65][66]. Lines with different MtNPDs mutation combinations presented smaller nodules, earlier nodule senescence, or ineffective nodules [67].
Nodule senescence can also be regulated by the genes associated with phytohormones and stress. Two genes ACS2 and ACO1, which encode two enzymes that are involved in the biosynthesis of ethylene, can activate nodule senescence [35]. Similarly, aldehyde oxidase AO3, which is a key enzyme for the biosynthesis of ABA, can also positively regulate nodule senescence [35]. Opposite to the above-mentioned three genes, GA2-oxidase, which is involved in the catabolism of gibberellins, has been shown to suppress nodule senescence [35]. Mt serpin6 and Mtferritins can mediate ordered drought-induced senescence and reduce plant growth [68]. MtCAS31 protects MtLb120-1 from the damage of drought stress to delay nodule senescence [69].
4. Abiotic Factors in the Regulation of Nodule Senescence
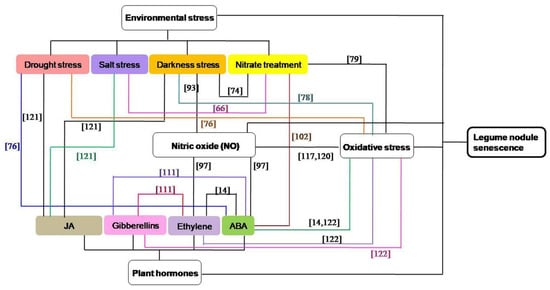
Nodule senescence is mainly triggered by normal development, while environmental stress can also affect and regulate its progress [71][74][75], such as drought [68][76], salt [62][77], darkness [71][78], nitrate [37][79][80][81], and so on. Under drought stress, more deleterious reactive oxygen species (ROS) are produced, and the synthesis of ferritin is inhibited, thus accelerating the senescence of root nodules [68], while leaves of low photosynthetic capacity are sacrificed in favor of nodule nitrogen metabolism to delay nodule senescence [76]. Salt stress can inhibit the N2-fixation activity and nodule respiration, and induce premature senescence of existing nodules [77]. Nitrogenase activity and the content of leghemoglobin are decreased under prolonged dark treatment, which can rapidly trigger a wide range of nodule senescence [71]. In nitrate-induced nodule senescence, the antioxidant content and nitrogenase activity are reduced, leading to serious impairment of nodule metabolism related to nodule senescence [79]. Besides, it has also been shown that the expression of acds is increased in nodules under hypoxic conditions, resulting in postponed degradation of enzymes associated with nitrogen fixation and delayed senescence of nodules [82].
Accumulating evidence suggests that oxidative damage exists in legume nodule senescence [14][78][83][84], and in these oxidative stress-induced aging nodules, ROS (mainly organic peroxides), oxidized glutathione and homoglutathione, catalytic Fe, lipid peroxidation, and oxidatively modified proteins and DNA bases are increased [84][85][86]. Oxidative stress is a result of over production of free radicals and is associated ROS [87]. ROS may induce irreversible posttranslational modification (carbonylation, advanced glycation) of crucial proteins in nodules, which play key roles in cell metabolism and nodule senescence [88]. Mitochondria are an important source of regulatory redox signals and an early target of oxidative modifications in senescing legume nodules [14][89]. ROS (such as H2O2) was shown to act as signaling molecule that regulate nodule senescence [14][31], and the concentrations of ROS will be tightly controlled by antioxidant enzymes and metabolites [90]. The antioxidant systems in symbiotic nodules can protect cells from oxidative damage [14][91][92], and the roles of ROS and antioxidant systems in nodule senescence have been reviewed comprehensively elsewhere [14][90].
As a signaling and defense molecule involved in diverse plant developmental processes and the plant response to pathogens, nitric oxide (NO) has been detected as a signal in nodule senescence [93]. Reducing the NO concentration can delay the senescence of nodules in dark conditions, while increasing the endogenous NO leads to cytological modifications of the nodule structure and the early expression of a specific senescence marker [93]. Meanwhile, the rhizobia can control NO-mediated post-translational modifications of legume proteins to regulate nodule senescence [93][94][95]. Hmp-a, encoding a NO detoxification enzyme, can inhibit the level of NO and delay nodule senescence [93][96]. Similarly, LjGlb1-1, a non-symbiotic class 1 Hb, can negatively regulate the level of NO, enhance the nitrogenase activity of mature and senescent nodules, and delay nodule senescence [97][98]. Meanwhile, iron-induced NO leads to an increased expression of ferritin during the senescence of L. japonicus nodules, supporting the above-mentioned point [99].
Nodule senescence is also tightly regulated by plant hormones, which are small molecules playing versatile roles in regulating plant growth, development, and responses to the environment [100]. These nodule senescence-related hormones mainly include ABA, ethylene, gibberellins, and jasmonate (JA). ABA plays roles in plants generally by decreasing growth rate and enhancing cell sustainability, while it also can inhibit lateral root development [101]. ABA is involved in the response of roots to high nitrate levels in the soil [102], and regulates the root nodule senescence [103]. When legumes are treated with ABA, the contents of leghemoglobin and N-fixation are declined, leading to the premature senescence of root nodules [103]. During nodule senescence, ethylene response factors (ERFs), SAM synthetase, ACC synthase, and ACC oxidase are up-regulated, which can increase ethylene production and activate nodule senescence [104][105]. Besides, when ethylene biosynthesis and signaling are altered, the bacteroid number per symbiosome [106], bacterial elongation [107] and bacteroid senescence will change [108]. Gibberellins are a large group of diterpenoid carboxylic acids in higher plants and have been well studied in nodulation, nodule development, and senescence [109][110][111]. The size of the nodule and the zone of nitrogen fixation are increased, and the number of nodules and senescence zone is decreased in the GA3-treated legumes [111], suggesting a negative effect of GAs on the nodule senescence. Furthermore, GA3 treatment can stimulate nodule meristem bifurcation [111], indicating a possible role of GAs in the redifferentiation of nodule meristem. JAs are important molecules in regulating physiological processes in plant growth and development, which can mediate the plant responses to biotic and abiotic stresses [112]. JAs are highly expressed in root nodules, and previous studies have shown that JAs can inhibit the expressions of early nodulation genes to negatively regulate nodulation [113]. Besides, they can also affect nodule cell growth and senescence process by affecting the antioxidant metabolism [114].
Among the above-mentioned factors, the concentration of NO in legumes will increase when the plants are exposed to environmental stresses [115][116][117]. NO may be produced downstream in the phytohormone signaling pathway in nodules [97], and plant hormones play critical roles in plant responses to adverse environmental conditions [100][118]. ROS and antioxidants can interact with hormones in the orchestration of nodule senescence [14], ROS and antioxidant systems are also participate in environmental stress-induced nodule senescence [76][78][79][119], and crosstalk between ROS and NO appears to be a key point to understand the redox regulation of symbiosis [120]. Therefore, these four signaling pathways can interact with each other in the regulating the senescence of nodules, and the interactions between different hormones or different environmental stresses are also existed during nodule senescence (Figure 2). In darkness-induced nodule senescence, the degree of senescence is reduced by removing NO from the nodules, suggesting that NO could be an intermediate in this process [93]. ABA and ethylene can induce NO accumulation during nodule senescence and the senescence-associated changes are suppressed by the NO scavenger cPTIO, indicating that ABA and ethylene trigger nodule senescence maybe by regulating the NO levels of nodules [97]. JAs, which have a strong senescence-promoting effect, were recognized as being signals in plant responses to lots of biotic and abiotic factors, including salt stress, drought stress and darkness stress [121]. ABA can interact with drought stress [76] or nitrate treatment [102] in the regulating the nodule senescence. The interactions between different hormones [14][111] or different environmental stress factors [62][74] are also found during nodule senescence. In drought stress [76], darkness stress [78] and nitrate treatment- included [79] nodule senescence, ROS and oxidatively modified proteins are increased, and these oxidative damage may have originated from the decrease in antioxidant defenses. Ascorbate, an important nodule antioxidant, is involved in dioxygenase reactions, such as those required for the synthesis of ABA, gibberellic acid and ethylene [14][122]. Both ROS and NO can mediate post-translational modification of proteins during nodule senescence, and legume plants possess different hemoglobins which play a significant role in ROS and RNS metabolism by contributing to ROS production and NO scavenging [117][120].
This entry is adapted from the peer-reviewed paper 10.3390/plants10061103
References
- Stonoha-Arther, C.; Wang, D. Tough love: Accommodating intracellular bacteria through directed secretion of antimicrobial peptides during the nitrogen-fixing symbiosis. Curr. Opin. Plant Biol. 2018, 44, 155–163.
- Biswas, B.; Gresshoff, P.M. The role of symbiotic nitrogen fixation in sustainable production of biofuels. Int. J. Mol. Sci. 2014, 15, 7380–7397.
- Zhang, X. Application of plant rhizobia inoculation in agriculture, forestry, animal husbandry and environmental protection. Biol. Teach. 2012, 37, 2–4.
- Li, X.; Xu, R.; Liao, H. Contribution and application potential of soybean symbiotic nitrogen fixation in reducing weight and increasing efficiency in agriculture. Soybean Sci. 2016, 35, 531–535.
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.H.; Lin, Y.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76.
- Tsyganova, A.V.; Kitaeva, A.B.; Tsyganov, V.E. Cell differentiation in nitrogen-fixing nodules hosting symbiosomes. Funct. Plant Biol. 2018, 45, 47–57.
- Via, V.D.; Zanetti, M.E.; Blanco, F. How legumes recognize rhizobia. Plant Signal. Behav. 2016, 11, e1120396.
- Timmers, A.C.; Auriac, M.C.; Truchet, G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 1999, 126, 3617–3628.
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate map of Medicago truncatula root nodules. Development 2014, 141, 3517–3528.
- Mergaert, P.; Kereszt, A.; Kondorosi, E. Gene Expression in Nitrogen-Fixing Symbiotic Nodule Cells in Medicago truncatula and Other Nodulating Plants. Plant Cell 2020, 32, 42–68.
- Hirsch, A.M. Tansley Review No. 40. Developmental Biology of Legume Nodulation. New Phytol. 1992, 122, 211–237.
- Vasse, J.; de Billy, F.; Camut, S.; Truchet, G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 1990, 172, 4295–4306.
- Kawaharada, Y.; Nielsen, M.W.; Kelly, S.; James, E.K.; Andersen, K.R.; Rasmussen, S.R.; Füchtbauer, W.; Madsen, L.H.; Heckmann, A.B.; Radutoiu, S.; et al. Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat. Commun. 2017, 8, 14534.
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; de Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume nodule senescence: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005, 165, 683–701.
- Navascués, J.; Pérez-Rontomé, C.; Gay, M.; Marcos, M.; Yang, F.; Walker, F.A.; Desbois, A.; Abián, J.; Becana, M. Leghemoglobin green derivatives with nitrated hemes evidence production of highly reactive nitrogen species during aging of legume nodules. Proc. Natl. Acad. Sci. USA 2012, 109, 2660–2665.
- Xia, X. Phenotypic Analysis and Molecular Markers of Plant Nodule Senescence. Methods Mol. Biol. 2018, 1744, 65–80.
- Wilson, D.O.; Reisenauer, H.M. Determination of leghemoglobin in legume nodules. Anal. Biochem. 1963, 6, 27–30.
- Schiffmann, J.; Löbel, R. Haemoglobin determination and its value as an early indication of peanut rhizobium efficiency. Plant Soil 1970, 33, 501–512.
- Van de Velde, W.; Guerra, J.C.; De Keyser, A.; De Rycke, R.; Rombauts, S.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Holsters, M.; Goormachtig, S. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol. 2006, 141, 711–720.
- Hernández-Jiménez, M.J.; Lucas, M.M.; Felipe, M.R.d. Antioxidant defence and damage in senescing lupin nodules. Plant Physiol. Biochem. 2002, 40, 645–657.
- Yuan, S.L.; Li, R.; Chen, H.F.; Zhang, C.J.; Chen, L.M.; Hao, Q.N.; Chen, S.L.; Shan, Z.H.; Yang, Z.L.; Zhang, X.J.; et al. RNA-Seq analysis of nodule development at five different developmental stages of soybean (Glycine max) inoculated with Bradyrhizobium japonicum strain 113-2. Sci. Rep. 2017, 7, 42248.
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501.
- Li, Y.; Zhou, L.; Li, Y.; Chen, D.; Tan, X.; Lei, L.; Zhou, J. A nodule-specific plant cysteine proteinase, AsNODF32, is involved in nodule senescence and nitrogen fixation activity of the green manure legume Astragalus sinicus. New Phytol. 2008, 180, 185–192.
- Haag, A.F.; Baloban, M.; Sani, M.; Kerscher, B.; Pierre, O.; Farkas, A.; Longhi, R.; Boncompagni, E.; Hérouart, D.; Dall’angelo, S.; et al. Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol. 2011, 9, e1001169.
- Bourcy, M.; Brocard, L.; Pislariu, C.I.; Cosson, V.; Mergaert, P.; Tadege, M.; Mysore, K.S.; Udvardi, M.K.; Gourion, B.; Ratet, P. Medicago truncatula DNF2 is a PI-PLC-XD-containing protein required for bacteroid persistence and prevention of nodule early senescence and defense-like reactions. New Phytol. 2013, 197, 1250–1261.
- Franck, S.; Strodtman, K.N.; Qiu, J.; Emerich, D.W. Transcriptomic Characterization of Bradyrhizobium diazoefficiens Bacteroids Reveals a Post-Symbiotic, Hemibiotrophic-Like Lifestyle of the Bacteria within Senescing Soybean Nodules. Int. J. Mol. Sci. 2018, 19, 3918.
- Strodtman, K.N.; Frank, S.; Stevenson, S.; Thelen, J.J.; Emerich, D.W. Proteomic Characterization of Bradyrhizobium diazoefficiens Bacteroids Reveals a Post-Symbiotic, Hemibiotrophic-Like Lifestyle of the Bacteria within Senescing Soybean Nodules. Int. J. Mol. Sci. 2018, 19, 3947.
- Hardy, R.W.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The acetylene-ethylene assay for n(2) fixation: Laboratory and field evaluation. Plant Physiol. 1968, 43, 1185–1207.
- Egli, M.A.; Griffith, S.M.; Miller, S.S.; Anderson, M.P.; Vance, C.P. Nitrogen Assimilating Enzyme Activities and Enzyme Protein during Development and Senescence of Effective and Plant Gene-Controlled Ineffective Alfalfa Nodules. Plant Physiol. 1989, 91, 898–904.
- Lodwig, E.M.; Hosie, A.H.; Bourdès, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 2003, 422, 722–726.
- Montiel, J.; Arthikala, M.K.; Cárdenas, L.; Quinto, C. Legume NADPH Oxidases Have Crucial Roles at Different Stages of Nodulation. Int. J. Mol. Sci. 2016, 17, 680.
- Nukui, N.; Ezura, H.; Minamisawa, K. Transgenic Lotus japonicus with an ethylene receptor gene Cm-ERS1/H70A enhances formation of infection threads and nodule primordia. Plant Cell Physiol. 2004, 45, 427–435.
- Yuhashi, K.; Ichikawa, N.; Ezura, H.; Akao, S.; Minakawa, Y.; Nukui, N.; Yasuta, T.; Minamisawa, K. Rhizobitoxine production by Bradyrhizobium elkanii enhances nodulation and competitiveness on Macroptilium atropurpureum. Appl. Environ. Microbiol. 2000, 66, 2658–2663.
- Heidstra, R.; Yang, W.C.; Yalcin, Y.; Peck, S.; Emons, A.M.; van Kammen, A.; Bisseling, T. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 1997, 124, 1781–1787.
- Serova, T.A.; Tikhonovich, I.A.; Tsyganov, V.E. Analysis of nodule senescence in pea (Pisum sativum L.) using laser microdissection, real-time PCR, and ACC immunolocalization. J. Plant Physiol. 2017, 212, 29–44.
- Maunoury, N.; Redondo-Nieto, M.; Bourcy, M.; Van de Velde, W.; Alunni, B.; Laporte, P.; Durand, P.; Agier, N.; Marisa, L.; Vaubert, D.; et al. Differentiation of symbiotic cells and endosymbionts in Medicago truncatula nodulation are coupled to two transcriptome-switches. PLoS ONE 2010, 5, e9519.
- Cabeza, R.; Koester, B.; Liese, R.; Lingner, A.; Baumgarten, V.; Dirks, J.; Salinas-Riester, G.; Pommerenke, C.; Dittert, K.; Schulze, J. An RNA sequencing transcriptome analysis reveals novel insights into molecular aspects of the nitrate impact on the nodule activity of Medicago truncatula. Plant Physiol. 2014, 164, 400–411.
- Chungopast, S.; Hirakawa, H.; Sato, S.; Handa, Y.; Saito, K.; Kawaguchi, M.; Tajima, S.; Nomura, M. Transcriptomic profiles of nodule senescence in Lotus japonicus and Mesorhizobium loti symbiosis. Plant Biotechnol. 2014, 31, 345–349.
- Yuan, S.; Ke, D.; Li, R.; Li, X.; Wang, L.; Chen, H.; Zhang, C.; Huang, Y.; Chen, L.; Hao, Q.; et al. Genome-wide survey of soybean papain-like cysteine proteases and their expression analysis in root nodule symbiosis. BMC Plant Biol. 2020, 20, 517.
- Yuan, S.; Li, R.; Wang, L.; Chen, H.; Zhang, C.; Chen, L.; Hao, Q.; Shan, Z.; Zhang, X.; Chen, S.; et al. Search for Nodulation and Nodule Development-Related Cystatin Genes in the Genome of Soybean (Glycine max). Front. Plant Sci. 2016, 7, 1595.
- Yuan, S.; Li, X.; Li, R.; Wang, L.; Zhang, C.; Chen, L.; Hao, Q.; Zhang, X.; Chen, H.; Shan, Z.; et al. Genome-Wide Identification and Classification of Soybean C2H2 Zinc Finger Proteins and Their Expression Analysis in Legume-Rhizobium Symbiosis. Front. Microbiol. 2018, 9, 126.
- Pladys, D.; Vance, C.P. Proteolysis during Development and Senescence of Effective and Plant Gene-Controlled Ineffective Alfalfa Nodules. Plant Physiol. 1993, 103, 379–384.
- Naito, Y.; Fujie, M.; Usami, S.; Murooka, Y.; Yamada, T. The involvement of a cysteine proteinase in the nodule development in Chinese milk vetch infected with Mesorhizobium huakuii subsp. rengei. Plant Physiol. 2000, 124, 1087–1096.
- Pierre, O.; Hopkins, J.; Combier, M.; Baldacci, F.; Engler, G.; Brouquisse, R.; Hérouart, D.; Boncompagni, E. Involvement of papain and legumain proteinase in the senescence process of Medicago truncatula nodules. New Phytol. 2014, 202, 849–863.
- Deng, J.; Zhu, F.; Liu, J.; Zhao, Y.; Wen, J.; Wang, T.; Dong, J. Transcription Factor bHLH2 Represses CYSTEINE PROTEASE77 to Negatively Regulate Nodule Senescence. Plant Physiol. 2019, 181, 1683–1703.
- Vincent, J.L.; Brewin, N.J. Immunolocalization of a cysteine protease in vacuoles, vesicles, and symbiosomes of pea nodule cells. Plant Physiol. 2000, 123, 521–530.
- Kardailsky, I.V.; Brewin, N.J. Expression of cysteine protease genes in pea nodule development and senescence. Mol. Plant Microbe Interact. 1996, 9, 689–695.
- Sheokand, S.; Dahiya, P.; Vincent, J.L.; Brewin, N.J. Modified expression of cysteine protease affects seed germination, vegetative growth and nodule development in transgenic lines of Medicago truncatula. Plant Sci. 2005, 169, 966–975.
- Alesandrini, F.; Mathis, R.; Sype, G.V.d.; Hérouart, D.; Puppo, A. Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytol. 2003, 158, 131–138.
- Yang, S.; Wang, Q.; Fedorova, E.; Liu, J.; Qin, Q.; Zheng, Q.; Price, P.A.; Pan, H.; Wang, D.; Griffitts, J.S.; et al. Microsymbiont discrimination mediated by a host-secreted peptide in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2017, 114, 6848–6853.
- Wang, Q.; Liu, J.; Li, H.; Yang, S.; Körmöczi, P.; Kereszt, A.; Zhu, H. Nodule-Specific Cysteine-Rich Peptides Negatively Regulate Nitrogen-Fixing Symbiosis in a Strain-Specific Manner in Medicago truncatula. Mol. Plant Microbe Interact. 2018, 31, 240–248.
- Wang, Q.; Yang, S.; Liu, J.; Terecskei, K.; Ábrahám, E.; Gombár, A.; Domonkos, Á.; Szűcs, A.; Körmöczi, P.; Wang, T.; et al. Host-secreted antimicrobial peptide enforces symbiotic selectivity in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2017, 114, 6854–6859.
- Kim, M.; Chen, Y.; Xi, J.; Waters, C.; Chen, R.; Wang, D. An antimicrobial peptide essential for bacterial survival in the nitrogen-fixing symbiosis. Proc. Natl. Acad. Sci. USA 2015, 112, 15238–15243.
- Horváth, B.; Domonkos, Á.; Kereszt, A.; Szűcs, A.; Ábrahám, E.; Ayaydin, F.; Bóka, K.; Chen, Y.; Chen, R.; Murray, J.D.; et al. Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc. Natl. Acad. Sci. USA 2015, 112, 15232–15237.
- Xi, J.; Chen, Y.; Nakashima, J.; Wang, S.M.; Chen, R. Medicago truncatula esn1 defines a genetic locus involved in nodule senescence and symbiotic nitrogen fixation. Mol. Plant Microbe Interact. 2013, 26, 893–902.
- Berrabah, F.; Bourcy, M.; Eschstruth, A.; Cayrel, A.; Guefrachi, I.; Mergaert, P.; Wen, J.; Jean, V.; Mysore, K.S.; Gourion, B.; et al. A nonRD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol. 2014, 203, 1305–1314.
- Krusell, L.; Krause, K.; Ott, T.; Desbrosses, G.; Krämer, U.; Sato, S.; Nakamura, Y.; Tabata, S.; James, E.K.; Sandal, N.; et al. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 2005, 17, 1625–1636.
- Hakoyama, T.; Niimi, K.; Yamamoto, T.; Isobe, S.; Sato, S.; Nakamura, Y.; Tabata, S.; Kumagai, H.; Umehara, Y.; Brossuleit, K.; et al. The integral membrane protein SEN1 is required for symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol. 2012, 53, 225–236.
- Kumagai, H.; Hakoyama, T.; Umehara, Y.; Sato, S.; Kaneko, T.; Tabata, S.; Kouchi, H. A novel ankyrin-repeat membrane protein, IGN1, is required for persistence of nitrogen-fixing symbiosis in root nodules of Lotus japonicus. Plant Physiol. 2007, 143, 1293–1305.
- Sinharoy, S.; Torres-Jerez, I.; Bandyopadhyay, K.; Kereszt, A.; Pislariu, C.I.; Nakashima, J.; Benedito, V.A.; Kondorosi, E.; Udvardi, M.K. The C2H2 transcription factor regulator of symbiosome differentiation represses transcription of the secretory pathway gene VAMP721a and promotes symbiosome development in Medicago truncatula. Plant Cell 2013, 25, 3584–3601.
- D’Haeseleer, K.; De Keyser, A.; Goormachtig, S.; Holsters, M. Transcription factor MtATB2: About nodulation, sucrose and senescence. Plant Cell Physiol. 2010, 51, 1416–1424.
- De Zélicourt, A.; Diet, A.; Marion, J.; Laffont, C.; Ariel, F.; Moison, M.; Zahaf, O.; Crespi, M.; Gruber, V.; Frugier, F. Dual involvement of a Medicago truncatula NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J. 2012, 70, 220–230.
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of Medicago truncatula seeds. Plant Physiol. 2013, 163, 757–774.
- Yamaya-Ito, H.; Shimoda, Y.; Hakoyama, T.; Sato, S.; Kaneko, T.; Hossain, M.S.; Shibata, S.; Kawaguchi, M.; Hayashi, M.; Kouchi, H.; et al. Loss-of-function of Aspartic Peptidase Nodule-Induced 1 (APN1) in Lotus japonicus restricts efficient nitrogen-fixing symbiosis with specific Mesorhizobium loti strains. Plant J. 2018, 93, 5–16.
- Domonkos, Á.; Kovács, S.; Gombár, A.; Kiss, E.; Horváth, B.; Kováts, G.Z.; Farkas, A.; Tóth, M.T.; Ayaydin, F.; Bóka, K.; et al. NAD1 Controls Defense-Like Responses in Medicago truncatula Symbiotic Nitrogen Fixing Nodules Following Rhizobial Colonization in a BacA-Independent Manner. Genes 2017, 8, 387.
- Wang, C.; Yu, H.; Luo, L.; Duan, L.; Cai, L.; He, X.; Wen, J.; Mysore, K.S.; Li, G.; Xiao, A.; et al. Nodules with Activated Defense 1 is required for maintenance of rhizobial endosymbiosis in Medicago truncatula. New Phytol. 2016, 212, 176–191.
- Trujillo, D.I.; Silverstein, K.A.T.; Young, N.D. Nodule-specific PLAT domain proteins are expanded in the Medicago lineage and required for nodulation. New Phytol. 2019, 222, 1538–1550.
- Dhanushkodi, R.; Matthew, C.; McManus, M.T.; Dijkwel, P.P. Drought-induced senescence of Medicago truncatula nodules involves serpin and ferritin to control proteolytic activity and iron levels. New Phytol. 2018, 220, 196–208.
- Li, X.; Feng, H.; Wen, J.; Dong, J.; Wang, T. MtCAS31 Aids Symbiotic Nitrogen Fixation by Protecting the Leghemoglobin MtLb120-1 Under Drought Stress in Medicago truncatula. Front. Plant Sci. 2018, 9, 633.
- Sueldo, D.J.; van der Hoorn, R.A.L. Plant life needs cell death, but does plant cell death need Cys proteases? FEBS J. 2017, 284, 1577–1585.
- Pérez Guerra, J.C.; Coussens, G.; De Keyser, A.; De Rycke, R.; De Bodt, S.; Van De Velde, W.; Goormachtig, S.; Holsters, M. Comparison of developmental and stress-induced nodule senescence in Medicago truncatula. Plant Physiol. 2010, 152, 1574–1584.
- Van Wyk, S.G.; Du Plessis, M.; Cullis, C.A.; Kunert, K.J.; Vorster, B.J. Cysteine protease and cystatin expression and activity during soybean nodule development and senescence. BMC Plant Biol. 2014, 14, 294.
- Young, N.D.; Debellé, F.; Oldroyd, G.E.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.; Gouzy, J.; Schoof, H.; et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011, 480, 520–524.
- Matamoros, M.A.; Baird, L.M.; Escuredo, P.R.; Dalton, D.A.; Minchin, F.R.; Iturbe-Ormaetxe, I.; Rubio, M.C.; Moran, J.F.; Gordon, A.J.; Becana, M. Stress-induced legume root nodule senescence. Physiological, biochemical, and structural alterations. Plant Physiol. 1999, 121, 97–112.
- Asensio, A.C.; Gil-Monreal, M.; Pires, L.; Gogorcena, Y.; Aparicio-Tejo, P.M.; Moran, J.F. Two Fe-superoxide dismutase families respond differently to stress and senescence in legumes. J. Plant Physiol. 2012, 169, 1253–1260.
- Marquez-Garcia, B.; Shaw, D.; Cooper, J.W.; Karpinska, B.; Quain, M.D.; Makgopa, E.M.; Kunert, K.; Foyer, C.H. Redox markers for drought-induced nodule senescence, a process occurring after drought-induced senescence of the lowest leaves in soybean (Glycine max). Ann. Bot. 2015, 116, 497–510.
- Swaraj, K.; Bishnoi, N.R. Effect of salt stress on nodulation and nitrogen fixation in legumes. Indian J. Exp. Biol. 1999, 37, 843–848.
- Gogorcena, Y.; Gordon, A.J.; Escuredo, P.R.; Minchin, F.R.; Witty, J.F.; Moran, J.F.; Becana, M. N2 Fixation, Carbon Metabolism, and Oxidative Damage in Nodules of Dark-Stressed Common Bean Plants. Plant Physiol. 1997, 113, 1193–1201.
- Escuredo, P.R.; Minchin, F.R.; Gogorcena, Y.; Iturbe-Ormaetxe, I.; Klucas, R.V.; Becana, M. Involvement of Activated Oxygen in Nitrate-Induced Senescence of Pea Root Nodules. Plant Physiol. 1996, 110, 1187–1195.
- Becana, M.; Aparicio-Tejo, P.M.; Sanchez-Diaz, M. Root Nodule Enzymes of Ammonia Metabolism from Medicago sativa L. as Influenced by Nitrate Levels. J. Plant Physiol. 1984, 116, 285–292.
- Chen, P.C.; Phillips, D.A. Induction of Root Nodule Senescence by Combined Nitrogen in Pisum sativum L. Plant Physiol. 1977, 59, 440–442.
- López, S.M.Y.; Sánchez, M.D.M.; Pastorino, G.N.; Franco, M.E.E.; García, N.T.; Balatti, P.A. Nodulation and Delayed Nodule Senescence: Strategies of Two Bradyrhizobium Japonicum Isolates with High Capacity to Fix Nitrogen. Curr. Microbiol. 2018, 75, 997–1005.
- Dalton, D.A.; Russell, S.A.; Hanus, F.J.; Pascoe, G.A.; Evans, H.J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc. Natl. Acad. Sci. USA 1986, 83, 3811–3815.
- Evans, P.J.; Gallesi, D.; Mathieu, C.; Hernandez, M.J.; Felipe, M.d.; Halliwell, B.; Puppo, A. Oxidative stress occurs during soybean nodule senescence. Planta 1999, 208, 73–79.
- Swaraj, K.; Dhandi, S.; Sheokand, S. Relationship between defense mechanism against activated oxygen species and nodule functioning with progress in plant and nodule development in Cajanus cajan L. Millsp. Plant Sci. 1995, 112, 65–74.
- Loscos, J.; Matamoros, M.A.; Becana, M. Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol. 2008, 146, 1282–1292.
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550.
- Matamoros, M.A.; Kim, A.; Peñuelas, M.; Ihling, C.; Griesser, E.; Hoffmann, R.; Fedorova, M.; Frolov, A.; Becana, M. Protein Carbonylation and Glycation in Legume Nodules. Plant Physiol. 2018, 177, 1510–1528.
- Matamoros, M.A.; Fernández-García, N.; Wienkoop, S.; Loscos, J.; Saiz, A.; Becana, M. Mitochondria are an early target of oxidative modifications in senescing legume nodules. New Phytol. 2013, 197, 873–885.
- Becana, M.; Matamoros, M.A.; Udvardi, M.; Dalton, D.A. Recent insights into antioxidant defenses of legume root nodules. New Phytol. 2010, 188, 960–976.
- Matamoros, M.A.; Dalton, D.A.; Ramos, J.; Clemente, M.R.; Rubio, M.C.; Becana, M. Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol. 2003, 133, 499–509.
- Redondo, F.J.; de la Peña, T.C.; Morcillo, C.N.; Lucas, M.M.; Pueyo, J.J. Overexpression of flavodoxin in bacteroids induces changes in antioxidant metabolism leading to delayed senescence and starch accumulation in alfalfa root nodules. Plant Physiol. 2009, 149, 1166–1178.
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric oxide (NO): A key player in the senescence of Medicago truncatula root nodules. New Phytol. 2012, 196, 548–560.
- Blanquet, P.; Silva, L.; Catrice, O.; Bruand, C.; Carvalho, H.; Meilhoc, E. Sinorhizobium meliloti Controls Nitric Oxide-Mediated Post-Translational Modification of a Medicago truncatula Nodule Protein. Mol. Plant Microbe Interact. 2015, 28, 1353–1363.
- Meilhoc, E.; Blanquet, P.; Cam, Y.; Bruand, C. Control of NO level in rhizobium-legume root nodules: Not only a plant globin story. Plant Signal. Behav. 2013, 8.
- Berger, A.; Boscari, A.; Frendo, P.; Brouquisse, R. Nitric oxide signaling, metabolism and toxicity in nitrogen-fixing symbiosis. J. Exp. Bot. 2019, 70, 4505–4520.
- Fukudome, M.; Watanabe, E.; Osuki, K.I.; Imaizumi, R.; Aoki, T.; Becana, M.; Uchiumi, T. Stably Transformed Lotus japonicus Plants Overexpressing Phytoglobin LjGlb1-1 Show Decreased Nitric Oxide Levels in Roots and Nodules as Well as Delayed Nodule Senescence. Plant Cell Physiol. 2019, 60, 816–825.
- Fukudome, M.; Calvo-Begueria, L.; Kado, T.; Osuki, K.; Rubio, M.C.; Murakami, E.; Nagata, M.; Kucho, K.; Sandal, N.; Stougaard, J.; et al. Hemoglobin LjGlb1-1 is involved in nodulation and regulates the level of nitric oxide in the Lotus japonicus-Mesorhizobium loti symbiosis. J. Exp. Bot. 2016, 67, 5275–5283.
- Chungopast, S.; Duangkhet, M.; Tajima, S.; Ma, J.F.; Nomura, M. Iron-induced nitric oxide leads to an increase in the expression of ferritin during the senescence of Lotus japonicus nodules. J. Plant Physiol. 2017, 208, 40–46.
- Jiang, K.; Asami, T. Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 1265–1300.
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555.
- Signora, L.; De Smet, I.; Foyer, C.H.; Zhang, H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. 2001, 28, 655–662.
- González, E.M.; Gálvez, L.; Arrese-Igor, C. Abscisic acid induces a decline in nitrogen fixation that involves leghaemoglobin, but is independent of sucrose synthase activity. J. Exp. Bot. 2001, 52, 285–293.
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99.
- Tittabutr, P.; Awaya, J.D.; Li, Q.X.; Borthakur, D. The cloned 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene from Sinorhizobium sp. strain BL3 in Rhizobium sp. strain TAL1145 promotes nodulation and growth of Leucaena leucocephala. Syst. Appl. Microbiol. 2008, 31, 141–150.
- Lohar, D.; Stiller, J.; Kam, J.; Stacey, G.; Gresshoff, P.M. Ethylene insensitivity conferred by a mutated Arabidopsis ethylene receptor gene alters nodulation in transgenic Lotus japonicus. Ann. Bot. 2009, 104, 277–285.
- Kondorosi, E.; Mergaert, P.; Kereszt, A. A paradigm for endosymbiotic life: Cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu. Rev. Microbiol. 2013, 67, 611–628.
- Guinel, F.C. Ethylene, a Hormone at the Center-Stage of Nodulation. Front. Plant Sci. 2015, 6, 1121.
- McAdam, E.L.; Reid, J.B.; Foo, E. Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. J. Exp. Bot. 2018, 69, 2117–2130.
- Hayashi, S.; Gresshoff, P.M.; Ferguson, B.J. Mechanistic action of gibberellins in legume nodulation. J. Integr. Plant Biol. 2014, 56, 971–978.
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins Inhibit Nodule Senescence and Stimulate Nodule Meristem Bifurcation in Pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 285.
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479.
- Costanzo, M.E.; Andrade, A.; del Carmen Tordable, M.; Cassán, F.; Abdala, G. Production and function of jasmonates in nodulated roots of soybean plants inoculated with Bradyrhizobium japonicum. Arch. Microbiol. 2012, 194, 837–845.
- Hause, B.; Schaarschmidt, S. The role of jasmonates in mutualistic symbioses between plants and soil-born microorganisms. Phytochemistry 2009, 70, 1589–1599.
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013, 201–202, 137–146.
- Nóra, L.; Lyudmila, L.; Peter, S.; Gábor, F.; Attila, Ö.; Kristóf, S.; László, E.; Zsuzsanna, K. Nitro-oxidative stress contributes to selenite toxicity in pea (Pisum sativum L.). Plant Soil 2016, 400, 107–122.
- Signorelli, S.; Sainz, M.; Tabares-da Rosa, S.; Monza, J. The Role of Nitric Oxide in Nitrogen Fixation by Legumes. Front. Plant Sci. 2020, 11, 521.
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86.
- Gogorcena, Y.; Iturbe-Ormaetxe, I.; Escuredo, P.R.; Becana, M. Antioxidant Defenses against Activated Oxygen in Pea Nodules Subjected to Water Stress. Plant Physiol. 1995, 108, 753–759.
- Puppo, A.; Pauly, N.; Boscari, A.; Mandon, K.; Brouquisse, R. Hydrogen peroxide and nitric oxide: Key regulators of the Legume-Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013, 18, 2202–2219.
- Wasternack, C.; Hause, B. Jasmonates and octadecanoids: Signals in plant stress responses and development. Prog. Nucleic Acid Res. Mol. Biol. 2002, 72, 165–221.
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta 2002, 1569, 1–9.
This entry is offline, you can click here to edit this entry!