The perinatal life is a critical period in the development and physiology of cardiac tissue. Higher cardiac energy metabolism is required in response to early postnatal oxygen levels, and increased cell proliferation is needed to attain normal neonatal growth and development [
12,
13,
14]. Cardiomyocyte regeneration is a highly energy-consuming process, and changes in energy metabolism happen as the neonatal cardiac development evolves rapidly within a short postnatal period; afterward, the cardiomyocytes exit the cell cycle. Fetal cardiomyocytes use glycolysis as the main source of energy during proliferation [
15,
16]. During early postnatal development, there is a shift from glycolysis to using fatty acid oxidation (FAO) [
17,
18], possibly due to increased energy demand during the transitioning from a fetal to postnatal period [
19], suggesting a correlation between cardiomyocyte proliferation and high oxidative energy metabolism. It is not clear whether this heart metabolic shift accounts for cardiomyocyte proliferation and hypertrophy or it is a consequence of increased oxygen availability in postnatal environment. Animal studies have shown that in younger animals, FAO facilitates cardiomyocyte proliferation and hypertrophic growth [
16]. Altered FAO could be associated with cardiac diseases [
20,
21]. Increased levels of oxygen during transition, from fetal to postnatal period, results in significantly increased oxidative stress as a result of the production of reactive oxygen species (ROS) with subsequent damage to cardiac tissue. These data could suggest that reducing the oxygen level and increased antioxidant levels might be useful to maintain the proliferative capacity of neonatal cardiomyocytes for a longer time [
12,
22]. These data suggest that the antioxidant could be used as a relevant therapy to extend the critical time window for cardiomyocyte proliferation by upregulating mitochondrial biogenesis and decrease mitophagy [
12,
23].
2.1. LCPUFAs and Cardiovascular Diseases
LCPUFAs are a group of essential fatty acids that can classify into two main categories: omega-3 (n-3) and omega-6 (n-6), depending on the position of the first double bond from the methyl end group of the fatty acid. Linoleic acid (LA; 18:2n-6) and α-linolenic acid (ALA; 18:3n-3) are the precursors of n-3 and n-6 LCPUFAs, respectively. The metabolic pathway involves a series of desaturase and elongase enzymes leading to the production of eicosapentaenoic acid (EPA; 22:5n-3) from ALA or arachidonic acid (ARA; 20:4n-6) from LA, based on the availability. EPA is then elongated and desaturated to produce docosahexaenoic acid (DHA; 22:6n-3).
Human studies have shown limited endogenous synthesis of DHA from n-3 fatty acid precursor ALA, necessitating the exogenous supplementation of a diet containing n-3 LCPUFAs for optimal health and development [
28,
29]. In adults, circulating EPA levels increased with DHA supplementation. However, ALA and EPA supplementation did not result in increased DHA levels in circulation [
29]. In contrast, Metherel et al. showed that supplemental EPA was converted to DHA [
30]. Increased levels of DHA to EPA could be either due to retro-conversion [
31,
32,
33] or reduced EPA metabolism [
34], suggesting that circulating levels of EPA and DHA are differently regulated.
A large, prospective, randomized clinical trial of 11,324 patients with a recent myocardial infarction showed a reduced relative risk of death in response to LCPUFAs [
35]. A lower rate of myocardial infarction was observed in people consuming an Inuit diet, a diet characterized by the high consumption of marine mammals and fish [
36]. The Inuit diet is rich in n-3 LCPUFAs, particularly EPA and DHA compared to those consuming a Western diet, which is abundant in n-6 LCPUFAs, mainly derived from vegetable oils rich in LA [
11,
37]. Furthermore, a significant difference was observed for cardiovascular mortality in two cohorts with n-3 versus n-6 LCPUFA enriched diets [
38,
39]. Clinical studies have shown a reduction in mortality of patients with cardiovascular diseases in response to n-3 LCPUFAs [
40,
41]. Meta-analyses in adults have shown that the Mediterranean diet, enriched in n-3 LCPUFAs [
42] and oleic acid [
43], has a protective effect against cardiovascular diseases [
43,
44,
45]. These studies declared the importance of bioactive compounds related to the diet. The beneficial effect of n-3 LCPUFAs (EPA and DHA) against cardiovascular diseases could be due to anti-arrhythmic, anti-inflammatory, anti-thrombotic, and hypolipidemic effects and improvement of vascular function [
46,
47]. However, a recent clinical trial failed to show a protective effect against arrhythmia in patients with myocardial infarction [
48]. Supplementation of n-3 LCPUFAs showed no effect on atrial fibrillation [
49], cardiac death, myocardial infarction, or stroke [
50].
In the Mediterranean diet, one of the main dietary source of n-3 LCPUFAs is fish, particularly oily fish species and shellfish [
51]. Neonates, specifically preterm infants, are at risk of a LCPUFA deficit, which has been associated with a subsequent increased risk of various neonatal diseases [
52]. Infants with heart disease may require longer parenteral nutrition during their hospitalization that fails to maintain the critical blood levels of LCPUFAs. In neonates, adequate nutritional intake of LCPUFAs is important for visual development, growth, complex brain function, and immune function [
53]. Parenteral administration of n-3 LCPUFAs containing lipid emulsion to infants previous to cardiac surgery decreased pro-inflammatory response after surgery [
54]. These data suggest the beneficial impact of LCPUFAs in infants with cardiac diseases [
52].
2.2. LCPUFAs Derived Mediators and Their Implication in Cardiovascular Physiology
LCPUFAs act as substrates for oxylipin production. LCPUFA metabolism to oxylipins occurs through three different pathways that are mediated by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 enzymes (CYP) as a result of transcellular biosynthesis and coordination between distinct cell types [
55,
56,
57]. Additionally, oxylipins can be produced by free radical catalyzed non-enzymatic lipid peroxidation [
58,
59]. High n-6 LCPUFAs and n-3 LCPUFAs intake is associated with higher levels of n-6 and n-3 LCPUFA-derived oxylipins, respectively [
55]. In recent years, an active role for to maintain the tissues homeostasis has been attributed to the local generation of specialized pro-resolving mediators (SPMs). SPMs exert their effect by interacting with G-protein coupled receptor (GPCR), such as ALX/FPR2, GPR32, ChemR23, BLT1, and GPR18 [
60,
61], and modulate diverse biological responses [
62] by regulating mitogen-activated protein kinase signaling, NF-κB pathways, AP-1 activation, and oxidative stress-related metabolism [
63]. SPMs suppress the expression of adhesion molecules on leukocytes, endothelial cells, neutrophil chemotaxis, and IL-8 production. Frequently, SPMs are rapidly inactivated locally by eicosanoid oxidoreductases and prostaglandin dehydrogenase [
64].
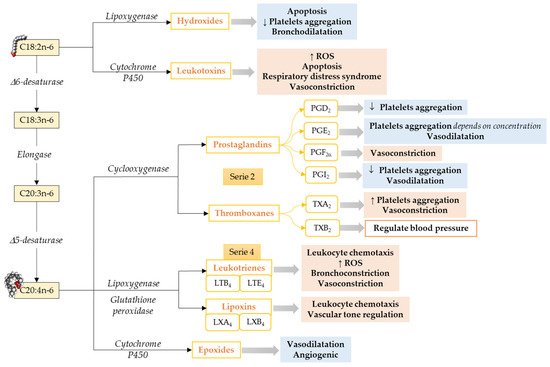
Omega-6 mediators. The most well-known oxylipins, the eicosanoids, are formed from ARA. ARA produces series-2 oxylipins via the COX pathway, resulting in the formation of prostaglandin G2 and subsequently to prostaglandin H2, which later gets converted to other prostaglandins and thromboxanes by specific prostaglandin and thromboxane synthases enzymes [
65] (). Prostaglandins make a huge oxylipins family with multiple biological functions such as inhibition of human platelet aggregation [
66,
67,
68], neutrophil degranulation [
69], innate immune response [
70], activation of plasminogen activator inhibitor type-1 [
71], and reducing pulmonary vascular resistance [
72]. Prostaglandins can either have pro-inflammatory and vasoconstrictor effects [
73] or anti-inflammatory and vasodilator effects [
67,
68], depending on to which receptor it binds [
74]. Thromboxane A2 is an endothelium-derived oxylipin that potentially induces vasoconstriction and platelet aggregation [
67] and thromboxane B2 is positively associated with high central blood pressure and multiple cardiovascular events [
75,
76].
Figure 1. Pathways to synthesize n-6 LCPUFA-derived lipid mediators and their functions focusing on the cardiovascular system and vascular endothelial function. C18:2n-6, Linoleic acid; C18:3n-6, γ-Linolenic acid; C20:3n-6; Dihomo-γ-Linolenic acid; C20:4n-6, Arachidonic acid; ROS, Reactive Oxidative Species. ↓ = Decreases; ↑ = Increases. Modified from [
55].
In addition to the COX pathway, oxylipins are also formed by a second pathway involving LOXs that catalyze the formation of hydroxy fatty acid (5-, 12-, and 15-HETE are the most commonly described [
77]) and their secondary metabolites, such as leukotrienes, lipoxins, resolvins, protectins, maresins, hepoxilins, and eoxins, via glutathione peroxidase [
77]. ARA, via the LOX pathway, produces HpETEs, which can be further converted to leukotrienes (series-4), which are associated with atherosclerosis, endothelial dysfunction, and cytokine release [
78]. Moreover, leukotrienes can also be converted to lipoxins [
57], which play a role in the resolution of inflammation [
79].
The third pathway that leads to oxylipins from LCPUFA metabolism involves a diverse array of membrane-bound cytochrome P450 (CYP) enzymes. ARA metabolism through CYP pathway activity results in the formation of HETE by CYP omega-hydroxylase activity. Cytochrome P450 can also act on ARA to synthesize epoxides, which could have vasodilator and vascular relaxing effects [
80].
Limited information is available about LA-derived oxylipins. LA produces oxylipins through the LOX pathway (i.e., 13-HODE), COX pathways (i.e., 9-HODE), and epoxygenase activity of CYP (i.e., EpOME), as well as non-enzymatical pathways (i.e., 9-HODE; ), and the relative importance of these pathways needs to be explored. LA-derived oxylipins have been shown to attenuate platelet adhesion to endothelial cells [
81], induce oxidative stress and a pro-inflammatory response in vascular endothelial cells [
82,
83], activate plasminogen activator inhibitor type-1 [
71], and prevent platelets from adhering to human vascular endothelial cells [
84,
85]. However, the direct effect of LA-derived oxylipins on the heart has not been studied.
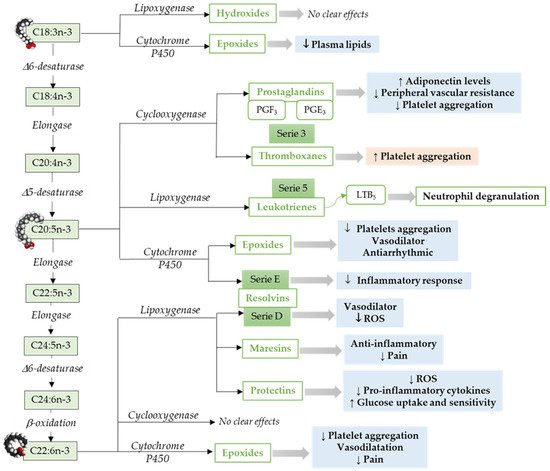
Omega-3 mediators. ALA, a precursor for n-3 LCPUFAs, can be metabolized to oxylipins by LOX [
86], COX [
55], or CYP cyclooxygenase activity [
87] (). ALA, metabolized via Cytochrome P450, results in epoxy fatty acids [
87], and the levels of these epoxy fatty acids were decreased in adults with hyperlipidemia [
88].
Figure 2. Pathways to synthesize n-3 LCPUFA-derived lipid mediators and its function focusing on the cardiovascular system and vascular endothelial function. C18:3n-3, α-Linolenic acid; C18:4n-3, Stearidonic acid; C20:4n-3, Eicosatetraenoic acid; C20:5n-3, Eicosapensaenoic acid; C22:5n-3, Docosapentaenoic acid; C24:5n-3, Tetracosapentaenoic acid; C24:6n-3, Tetracosahexaenoic acid; C22:5n-3, Docosahexaenoic acid; ROS, Reactive Oxidative Species. ↓ = Decreases; ↑ = Increases. Modified from [
55].
Similar to ARA, EPA metabolism through COX pathways generates oxylipins that includes series-3 prostaglandins (i.e., PGE3) and thromboxanes (i.e., thromboxane A3) [
55]. However, relative to ARA, EPA is a weak substrate for COX enzyme [
89]. Furthermore, EPA via LOX pathway results in leukotrienes (series-5) [
90]. An in vitro study has shown that leukotrienes derived from both n-6 and n-3 LCPUFAs induce neutrophil lysosomal degranulation. However, n-3 LCPUFA-derived leukotriene (leukotriene B5) is less effective compared to n-6 LCPUFA-derived leukotriene (leukotriene B4) [
91]. In addition, leukotriene B5 could be less inflammatory than leukotrienes 4-series [
92]. The epoxides-fatty acids derived from EPA via CYP pathways [
93], inhibits platelet aggregation, affect vasodilation, and have an antiarrhythmic effect in neonatal cardiomyocytes [
94,
95]. E-series resolvins synthesized by cytochrome P450 from EPA [
64,
96] have been shown to reduce neutrophil migration and inflammatory responses [
97].
In addition to EPA, DHA is the other predominant omega-3 LCPUFA. DHA metabolism through LOX pathway leads to the production of maresins, resolvins (D-series), and protectins [
55]. These endogenous lipid mediators including lipoxins, among other SPMs [
95]. DHA-derived 4-hydroxy-docosahexaenoic acid (14-OH-22:6) potentially inhibits platelet aggregation [
98]. Resolvins play an important role in inflammation, vascular biology, and platelet aggregation [
99,
100]. DHA-derived oxylipins can also be synthesized by COX [
101,
102] and cytochrome P450 epoxygenase. DHA derived epoxy-fatty acids [
93] are known to decrease platelet aggregation and thromboxane A2 synthesis [
95,
103].
In general, oxylipins generated from n-3 LCPUFAs have lesser effectiveness compared to those derived from n-6 LCPUFAs. The effect of n-3 LCPUFA-derived oxylipins could be antagonized by oxylipins derived from n-6 LCPUFAs [
104]. The n-3 LCPUFAs often compete with n-6 LCPUFAs for the same receptor and enzyme. The degree of membrane LCPUFAs incorporation might play an important role in determining the biological effect. Moreover, the enzyme activity could be regulated by increasing or decreasing the initial substrate within a given pathway.