Lactic acid bacteria (LAB) are generally accepted as safe microorganisms playing important roles in food fermentation and preservation, either by the presence of natural microbiota or through the addition of starter cultures under controlled conditions. The preservation effect exerted by LAB is mainly due to the production of lactic and acetic acids but also due to several other antimicrobial compounds with important anti-bacterial effect. Fruit and vegetables are an important part of human diets and provide multiple health benefits. However, due to the short shelf-life of fresh and minimally-processed fruit and vegetables, significant losses occur throughout the food distribution chain. Shelf-life extension requires preserving both the quality and safety of food products. Increasing the shelf-life of fresh and fresh-cut fruit and vegetables is challenging, but can be achieved by physical, chemical or biological methods. Foodborne diseases are a growing public health problem worldwide, particularly for infants, children and the elderly. Consumption of fresh fruits and vegetables is associated with a growing number of foodborne outbreaks due to bacterial contamination of these products. Postharvest management of fresh fruits and vegetables storage can be achieved through the use of lactic acid bacteria.
- postharvest management
- lactic acid bacteria
- fresh fruits and vegetables
- food safety
- foodborne bacterial pathogens
- natural antimicrobials
- edible coatings
- bacteriocin
- biopreservatives
- antimicrobial efficacy
Definition
The antibacterial effect of lactic acid bacteria is attributed to its ability to produce antimicrobial compounds, including bacteriocins, with strong competitive action against many microorganisms. The use of bacteriocins, both separately and in combination with edible coatings, is considered a very promising approach for microbiological quality, and safety for postharvest storage of raw and minimally processed fruits and vegetables.
Introduction
Data, Model, Applications and Influences
Lactic Acid Bacteria
The use of LAB play a dominant role in the fermentation of both food and feed [31], with health and nutritional benefits, and a very long history and safe use after consumption of fermented foods and beverages [32]. Taste and texture are the main (quality) characteristics of fermented foods that are enhanced with the addition of LAB [33]. Dairy products, fermented fruits and vegetables, meat-based products, and fermented beverages are the main fermented foods that involve LAB [34,35,36]. LAB exists in environments such as water, soil, sewage, plants, as well as in humans and animals [32]. In general, environments rich in available carbohydrates are ideal for the growth of LAB. Cavities of humans and animals are also favorable places for their growth [34]. LAB can be isolated from many raw fruits and vegetables, and then used against natural microbial populations [37].
LAB belong to different taxonomic groups of Gram-positive bacteria, with a common characteristic that produces lactic acid as the main (or sole) product during fermentation of carbohydrates [38,39]. They have rod- or coccus-shaped cells [40], do not form spores, and are anaerobic or microaerophilic and acid-tolerant organisms [41]. They are naturally present in several food products, from which can be isolated [42].
LAB are generally regarded as safe (GRAS) microorganisms by the United States Food and Drug Administration (FDA), and Qualified Presumption of Safety (QPS) by the European Food Safety Authority (EFSA). Their use in food biopreservation are considered an alternative for the prevention of the growth of pathogenic microorganisms [43,44], as their competitiveness against pathogenic microorganisms make them extremely ideal candidates for the development of bioprotective agents for fresh fruits and vegetables [45]. During biopreservation, either antimicrobial metabolites can be applied without the producing strain, or culture-producing antimicrobial metabolites can be added [46]. These starter cultures can be added, either as individual cultures or as multi-species consortia [47]. In the group of LAB bacteria, there are 6 families, 38 genera, and all belong to the Lactobacillales order, Bacilli class, and Firmicutes phylum.
Lactococcus, Streptococcus, Lactobacillus, Pediococcus, Leuconostoc, Lactosphaera, Melissococcus, Microbacterium, Propionibacterium, Enterococcus, Carnobacterium, Tetragenococcus, Aerococcus, Alloiococcus, Oenococcus, Vagococcus, Dolosigranulum, and Weisella are the most common genera that belong to LAB [48,49,50,51]. Among all the genes present in LAB, Lactobacillus consists of 261 species (March 2020), ranking it in the genus with the most members. The genus Lactobacillus has been reclassified into 25 genera, including the Lactobacillus delbrueckii group, Paralactobacillus, and 23 novel genera with the names Holzapfelia, Amylolactobacillus, Bombilactobacillus, Companilactobacillus, Lapidilactobacillus, Agrilactobacillus, Schleiferilactobacillus, Loigolactobacilus, Lacticaseibacillus, Latilactobacillus, Dellaglioa, Liquorilactobacillus, Ligilactobacillus, Lactiplantibacillus, Furfurilactobacillus, Paucilactobacillus, Limosilactobacillus, Fructilactobacillus, Acetilactobacillus, Apilactobacillus, Levilactobacillus, Secundilactobacillus and Lentilactobacillus [52]. The following are the most common species: Lactobacillus acidophilus, L. plantarum, L. Casei, L. rhamnosus, L. delbrueckii bulgaricus, L. fermentum, L. reuteri, Lactococcus lactis, Lactococcus lactis cremoris, Bifidobacterium bifidum, B. infantis, B. adolecentis, B. longum, B. breve, Enterococcus faecalis, Enterococcus faecium [51].
LAB produce a variety of antimicrobial compounds, such as organic acids (lactic, citric, acetic, fumaric, and malic acid), hydrogen peroxide, CO2, diacetyl, ethanol, reuterin, acetaldehyde, acetoin, ammonia, bacteriocins, bacteriocin-like inhibitory substances (BLIS), and other important metabolites, which possess strong antagonistic activity against many microorganisms [53,54,55,56] (Figure 1). In addition, the antimicrobial effect of lactic acid bacteria is the result of competition with pathogenic microorganisms for nutrients [24].
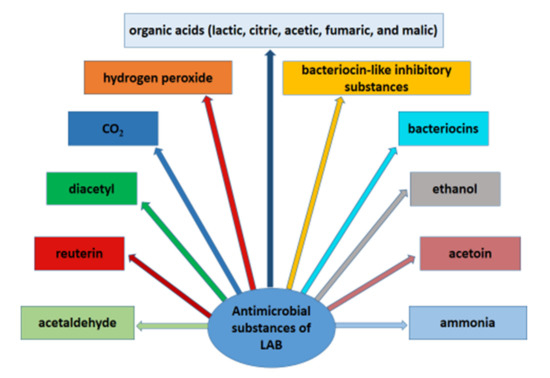 Figure 1. Antimicrobial substances produced by lactic acid bacteria.
Additionally, health-promoting properties have been linked with the presence of some strains of LAB and probiotics [57,58,59] as they have managed to reduce the risk of various diseases [60]. Probiotics have been identified as living microorganisms that have beneficial effects for humans and animals after adequate intake [61]. Probiotics have been used to prevent colon cancer [62], antibiotic-associated diarrhea, cholesterol reduction, lactose digestion [59], inflammatory bowel disease, breast cancer, and ulcerative colitis [63]. The genus Lactobacillus is one of the most widely used probiotics available on the market [60]. Probiotic bacteria do not live apart from the environment, but interact with the host, forming cooperative communities called biofilms [62].
In exception for their antimicrobial activity, LAB also have antifungal activity, which is of great interest, both against mycotoxigenic fungi and fungal mycotoxins, showing their potential by inactivation, removal, or detoxification processes [64,65,66]. The antifungal activity of LAB has prolonged the shelf life of fresh vegetables [67] and fruits [68].
Conclusions
Figure 1. Antimicrobial substances produced by lactic acid bacteria.
Additionally, health-promoting properties have been linked with the presence of some strains of LAB and probiotics [57,58,59] as they have managed to reduce the risk of various diseases [60]. Probiotics have been identified as living microorganisms that have beneficial effects for humans and animals after adequate intake [61]. Probiotics have been used to prevent colon cancer [62], antibiotic-associated diarrhea, cholesterol reduction, lactose digestion [59], inflammatory bowel disease, breast cancer, and ulcerative colitis [63]. The genus Lactobacillus is one of the most widely used probiotics available on the market [60]. Probiotic bacteria do not live apart from the environment, but interact with the host, forming cooperative communities called biofilms [62].
In exception for their antimicrobial activity, LAB also have antifungal activity, which is of great interest, both against mycotoxigenic fungi and fungal mycotoxins, showing their potential by inactivation, removal, or detoxification processes [64,65,66]. The antifungal activity of LAB has prolonged the shelf life of fresh vegetables [67] and fruits [68].
Conclusions
After harvesting fruits and vegetables and during storage and transportation, their sensorial, nutritional and sensorial quality decreases due to high moisture content, microbial growth, environmental factors, maturity and senescence. Considering their very short shelf life, fruits and vegetables need immediate post-harvest care to increase it. Biopreservation using LAB has been gaining interest, since they are classified as “generally recognized as safe” and have shown antimicrobial capacities; additionally, it is considered an environmentally friendly method. The use of a variety of antimicrobial compounds produced by LAB promises to be an effective technique to guarantee safety and to extend the useful life of fruits and vegetables during post-harvest period. The use of LAB in preservation of fresh fruits and vegetables, need further research. The ecological role of LAB is still under investigation in nature, but they can be considered as a sustainable option for preservation of fresh fruits and vegetables.
References
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Kowal, P.; Soriano, M.M.; Williams, S.; Banks, E.; Vo, K.; Byles, J. Fruit and vegetable intake and body mass index in a large sample of middle-aged Australian men and women. Nutrients 2014, 6, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- More, A.S.; Ranadheera, C.S.; Fang, Z.; Warner, R.; Ajlouni, S. Biomarkers associated with quality and safety of fresh-cut produce. Food Biosci. 2020, 34, 100524. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, Q.; Quentin, B.; Assemat, S.; Hoarau, M.; Meile, J.-C.; Remize, F. Changes of quality of minimally-processed pineapple (Ananas comosus, var. ‘Queen Victoria’) during cold storage: Fungi in the leading role. Microorganisms 2020, 8, 185. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 6, 940–975. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced lactic acid bacteria to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, E.; Abdalla, N.A.; Taha, H.S.; Fari, M. Postharvest management of fruits and vegetables storage. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 65–152. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services: Washington, DC, USA; U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 12 May 2020).
- IFPA (International Fresh-Cut Produce Association); PMA (The Produce Marketing Association). Handling Guidelines for the Fresh-Cut Produce Industry, 3rd ed.; IFPA: Alexandria, VA, USA, 1999; p. 5. [Google Scholar]
- Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A.; Sinigaglia, M. Non-conventional tools to preserve and prolong the quality of minimally-processed fruits and vegetables. Coatings 2015, 5, 931–961. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, C.Y.; Saltveit, M. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. In United States Department of Agriculture (USDA)—Agricultural Research Service–Agriculture Handbook; Nο 66; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2016; p. 780. Available online: http://www.ars.usda.gov/is/np/indexpubs (accessed on 12 May 2020).
- De Oliveira, P.M.; Leite Júnior, B.R.; Martins, M.L.; Martins, E.M.F.; Ramos, A.M. Minimally processed yellow melon enriched with probiotic bacteria. Semin. Agrar. 2014, 35, 2415–2426. [Google Scholar] [CrossRef]
- Salazar, J.K.; Sahu, S.N.; Hildebrandt, I.M.; Zhang, L.; Qi, Y.; Liggans, G.; Datta, A.R.; Tortorello, M.L. Growth kinetics of listeria monocytogenes in cut produce. J. Food Prot. 2017, 80, 1328–1336. [Google Scholar] [CrossRef]
- Ali, A.; Yeoh, W.K.; Forney, C.; Siddiqui, M.W. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulou, E.; Varzakas, T. Application of antibrowning agents in minimally processed cabbage. J. Food Nutr. Disord. 2014, 3, 2. [Google Scholar]
- Varzakas, T.; Manolopoulou, E. Comparison of HACCP and ISO 22000 in the ready-to-eat fruit and vegetable industry in conjunction with application of failure mode and effect analysis (FMEA) and Ishikawa diagrams. In Minimally Processed and Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 685–721. [Google Scholar]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of Golden Delicious apples and Iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Navarro-Rico, J.; Gómez, P.A.; Otón, M.; Artés, F.; Artés-Hernández, F. Combined sustainable sanitising treatments to reduce Escherichia coli and Salmonella Enteritidis growth on fresh-cut kailan-hybrid broccoli. Food Control 2015, 47, 312–317. [Google Scholar] [CrossRef]
- Asare, P.T.; Greppi, A.; Stettler, M.; Schwab, C.; Stevens, M.J.A.; Lacroix, C. Decontamination of minimally-processed fresh lettuce using reuterin produced by Lactobacillus reuteri. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio-control agents to improve the safety, shelf life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Sachadyn-król, M.; Agriopoulou, S. Ozonation as a method of abiotic elicitation improving the health-promoting properties of plant products—A review. Molecules 2020, 25, 2416. [Google Scholar] [CrossRef]
- Manolopoulou, E.; Varzakas, T. Minimally processed (fresh-cut) fruits and vegetables. In Handbook of Food Processing: Food Safety, Quality and Manufacturing Processes Contemporary Food Engineering Series; Sun, D.-W., Varzakas, T., Tzia, C., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2016; pp. 231–282. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Tumbarski, Y.; Nikolova, R.; Petkova, N.; Ivanov, I.; Lante, A. Biopreservation of fresh strawberries by carboxymethyl cellulose edible coatings enriched with a bacteriocin from Bacillus methylotrophicus BM47. Food Technol. Biotechnol. 2019, 57, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Leneveu-Jenvrin, C.; Charles, F.; Barba, F.J.; Remize, F. Role of biological control agents and physical treatments in maintaining the quality of fresh and minimally-processed fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, B.V.C.; Tandon, R.; Kapoor, S.; Sidhu, M.K. Natural coatings for shelf life enhancement and quality maintenance of fresh fruits and vegetables—A review. J. Postharvest Technol. 2018, 6, 12–26. [Google Scholar]
- Yépez, A.; Luz, C.; Meca, G.; Vignolo, G.; Mañes, J.; Aznar, R. Biopreservation potential of lactic acid bacteria from Andean fermented food of vegetal origin. Food Control 2017, 78, 393–400. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- Devi, M.; Jeyanthi Rebecca, L.; Sumathy, S. Bactericidal activity of the lactic acid bacteria Lactobacillus delbreukii. J. Chem. Pharm. Res. 2013, 5, 176–180. [Google Scholar]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Lactic Acid Bacteria; Zhang, Y., Cai, Y., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Kazakos, S.; Mantzourani, I.; Nouska, C.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S.; Varzakas, T. Production of low-alcohol fruit beverages through fermentation of pomegranate and orange juices with kefir grains. Curr. Res. Nutr. Food Sci. 2016, 4, 19–26. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Zakynthinos, G.; Proestos, C.; Radwanska, M. Fermented vegetables. In Minimally Processed and Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 537–584. [Google Scholar]
- Wu, R.; Lu, J. Proteomics of Lactic Acid Bacteria. In Lactic Acid Bacteria; Zhang, Y., Cai, Y., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; pp. 249–301. [Google Scholar]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo[a]pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Linares-Morales, J.R.; Gutiérrez-Méndez, N.; Rivera-Chavira, B.E.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018, 2, 50. [Google Scholar] [CrossRef]
- Muccilli, S.; Restuccia, C. Bioprotective Role of yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef]
- Sanpa, S.; Sanpa, S.; Suttajit, M. Lactic acid bacteria isolates from Pla-som, their antimicrobial activities and fermentation properties in Pla-som. J. Food Health Bioenviron. Sci. 2019, 12, 36–43. [Google Scholar]
- Singh, V.P. Recent approaches in food bio-preservation-A review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Khalid, K. An overview of lactic acid bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Djadouni, F.; Kihal, M. Antimicrobial activity of lactic acid bacteria and the spectrum of their biopeptides against spoiling germs in foods. Braz. Arch. Biol. Technol. 2012, 55, 435–444. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Zehra, S.A.; Javed, S.; Nadeem, S.G.; Hakim, S.T. Lactic acid bacteria from fresh fruits and vegetables as biocontrol agent of foodborne bacterial pathogens. RADS J. Biol. Res. Appl. Sci. 2014, 5, 36–45. [Google Scholar]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikien, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Cálix-Lara, T.F.; Rajendran, M.; Talcott, S.T.; Smith, S.B.; Mille, R.K.; Castillo, A.; Sturino, J.M.; Taylor, T.M. Inhibition of Escherichia coli O157: H7 and Salmonella enterica on spinach and identification of antimicrobial substances produced by a commercial lactic acid bacteria food safety intervention. Food Microbiol. 2014, 38, 192–200. [Google Scholar] [CrossRef]
- Malik, D.K.; Bhatia, D.; Nimbriya, A.; Kumar, S. Lactic acid bacteria and bacteriocin: A Review. J. Pharm. Res. 2012, 5, 2510–2513. [Google Scholar]
- Stefanis, C.; Mantzourani, I.; Plessas, S.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Kandylis, P.; Varzakas, T. Reviewing classical and molecular techniques regarding profiling of probiotic character of microorganisms. Curr. Res. Nutr. Food Sci. 2016, 4, 27–47. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of milk type on the microbiological, physicochemical and sensory characteristics of probiotic fermented milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO, Food and Nutritional Paper No. 85; FAO: Rome, Italy, 2006; pp. 1–50. [Google Scholar]
- Ayala, F.R.; Bauman, C.; Cogliati, S.; Leñini, C.; Bartolini, M.; Grau, R. Microbial flora, probiotics, Bacillus subtilis and the search for a long and healthy human longevity. Microbial. Cell. 2017, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020, 140, 103931. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Blagojev, N.; Škrinjar, M.; Vesković-Moračanin, S.; Šošo, V. Control of mould growth and mycotoxin production by lactic acid bacteria metabolites. Rom. Biotechnol. Lett. 2012, 17, 7219–7226. [Google Scholar]
- Sathe, S.J.; Nawani, N.N.; Dhakephalkar, P.K.; Kapadnis, B.P. Antifungal lactic acid bacteria with potential to prolong shelf life of fresh vegetables. J. Appl. Microbiol. 2007, 103, 2622–2628. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
Lactic Acid Bacteria

After harvesting fruits and vegetables and during storage and transportation, their sensorial, nutritional and sensorial quality decreases due to high moisture content, microbial growth, environmental factors, maturity and senescence. Considering their very short shelf life, fruits and vegetables need immediate post-harvest care to increase it. Biopreservation using LAB has been gaining interest, since they are classified as “generally recognized as safe” and have shown antimicrobial capacities; additionally, it is considered an environmentally friendly method. The use of a variety of antimicrobial compounds produced by LAB promises to be an effective technique to guarantee safety and to extend the useful life of fruits and vegetables during post-harvest period. The use of LAB in preservation of fresh fruits and vegetables, need further research. The ecological role of LAB is still under investigation in nature, but they can be considered as a sustainable option for preservation of fresh fruits and vegetables.
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Kowal, P.; Soriano, M.M.; Williams, S.; Banks, E.; Vo, K.; Byles, J. Fruit and vegetable intake and body mass index in a large sample of middle-aged Australian men and women. Nutrients 2014, 6, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- More, A.S.; Ranadheera, C.S.; Fang, Z.; Warner, R.; Ajlouni, S. Biomarkers associated with quality and safety of fresh-cut produce. Food Biosci. 2020, 34, 100524. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, Q.; Quentin, B.; Assemat, S.; Hoarau, M.; Meile, J.-C.; Remize, F. Changes of quality of minimally-processed pineapple (Ananas comosus, var. ‘Queen Victoria’) during cold storage: Fungi in the leading role. Microorganisms 2020, 8, 185. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 6, 940–975. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced lactic acid bacteria to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, E.; Abdalla, N.A.; Taha, H.S.; Fari, M. Postharvest management of fruits and vegetables storage. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 65–152. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services: Washington, DC, USA; U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 12 May 2020).
- IFPA (International Fresh-Cut Produce Association); PMA (The Produce Marketing Association). Handling Guidelines for the Fresh-Cut Produce Industry, 3rd ed.; IFPA: Alexandria, VA, USA, 1999; p. 5. [Google Scholar]
- Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A.; Sinigaglia, M. Non-conventional tools to preserve and prolong the quality of minimally-processed fruits and vegetables. Coatings 2015, 5, 931–961. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, C.Y.; Saltveit, M. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. In United States Department of Agriculture (USDA)—Agricultural Research Service–Agriculture Handbook; Nο 66; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2016; p. 780. Available online: http://www.ars.usda.gov/is/np/indexpubs (accessed on 12 May 2020).
- De Oliveira, P.M.; Leite Júnior, B.R.; Martins, M.L.; Martins, E.M.F.; Ramos, A.M. Minimally processed yellow melon enriched with probiotic bacteria. Semin. Agrar. 2014, 35, 2415–2426. [Google Scholar] [CrossRef]
- Salazar, J.K.; Sahu, S.N.; Hildebrandt, I.M.; Zhang, L.; Qi, Y.; Liggans, G.; Datta, A.R.; Tortorello, M.L. Growth kinetics of listeria monocytogenes in cut produce. J. Food Prot. 2017, 80, 1328–1336. [Google Scholar] [CrossRef]
- Ali, A.; Yeoh, W.K.; Forney, C.; Siddiqui, M.W. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulou, E.; Varzakas, T. Application of antibrowning agents in minimally processed cabbage. J. Food Nutr. Disord. 2014, 3, 2. [Google Scholar]
- Varzakas, T.; Manolopoulou, E. Comparison of HACCP and ISO 22000 in the ready-to-eat fruit and vegetable industry in conjunction with application of failure mode and effect analysis (FMEA) and Ishikawa diagrams. In Minimally Processed and Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 685–721. [Google Scholar]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of Golden Delicious apples and Iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Navarro-Rico, J.; Gómez, P.A.; Otón, M.; Artés, F.; Artés-Hernández, F. Combined sustainable sanitising treatments to reduce Escherichia coli and Salmonella Enteritidis growth on fresh-cut kailan-hybrid broccoli. Food Control 2015, 47, 312–317. [Google Scholar] [CrossRef]
- Asare, P.T.; Greppi, A.; Stettler, M.; Schwab, C.; Stevens, M.J.A.; Lacroix, C. Decontamination of minimally-processed fresh lettuce using reuterin produced by Lactobacillus reuteri. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio-control agents to improve the safety, shelf life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Sachadyn-król, M.; Agriopoulou, S. Ozonation as a method of abiotic elicitation improving the health-promoting properties of plant products—A review. Molecules 2020, 25, 2416. [Google Scholar] [CrossRef]
- Manolopoulou, E.; Varzakas, T. Minimally processed (fresh-cut) fruits and vegetables. In Handbook of Food Processing: Food Safety, Quality and Manufacturing Processes Contemporary Food Engineering Series; Sun, D.-W., Varzakas, T., Tzia, C., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2016; pp. 231–282. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Tumbarski, Y.; Nikolova, R.; Petkova, N.; Ivanov, I.; Lante, A. Biopreservation of fresh strawberries by carboxymethyl cellulose edible coatings enriched with a bacteriocin from Bacillus methylotrophicus BM47. Food Technol. Biotechnol. 2019, 57, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Leneveu-Jenvrin, C.; Charles, F.; Barba, F.J.; Remize, F. Role of biological control agents and physical treatments in maintaining the quality of fresh and minimally-processed fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, B.V.C.; Tandon, R.; Kapoor, S.; Sidhu, M.K. Natural coatings for shelf life enhancement and quality maintenance of fresh fruits and vegetables—A review. J. Postharvest Technol. 2018, 6, 12–26. [Google Scholar]
- Yépez, A.; Luz, C.; Meca, G.; Vignolo, G.; Mañes, J.; Aznar, R. Biopreservation potential of lactic acid bacteria from Andean fermented food of vegetal origin. Food Control 2017, 78, 393–400. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- Devi, M.; Jeyanthi Rebecca, L.; Sumathy, S. Bactericidal activity of the lactic acid bacteria Lactobacillus delbreukii. J. Chem. Pharm. Res. 2013, 5, 176–180. [Google Scholar]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Lactic Acid Bacteria; Zhang, Y., Cai, Y., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Kazakos, S.; Mantzourani, I.; Nouska, C.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S.; Varzakas, T. Production of low-alcohol fruit beverages through fermentation of pomegranate and orange juices with kefir grains. Curr. Res. Nutr. Food Sci. 2016, 4, 19–26. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Zakynthinos, G.; Proestos, C.; Radwanska, M. Fermented vegetables. In Minimally Processed and Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 537–584. [Google Scholar]
- Wu, R.; Lu, J. Proteomics of Lactic Acid Bacteria. In Lactic Acid Bacteria; Zhang, Y., Cai, Y., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; pp. 249–301. [Google Scholar]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo[a]pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Linares-Morales, J.R.; Gutiérrez-Méndez, N.; Rivera-Chavira, B.E.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018, 2, 50. [Google Scholar] [CrossRef]
- Muccilli, S.; Restuccia, C. Bioprotective Role of yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef]
- Sanpa, S.; Sanpa, S.; Suttajit, M. Lactic acid bacteria isolates from Pla-som, their antimicrobial activities and fermentation properties in Pla-som. J. Food Health Bioenviron. Sci. 2019, 12, 36–43. [Google Scholar]
- Singh, V.P. Recent approaches in food bio-preservation-A review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Khalid, K. An overview of lactic acid bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Djadouni, F.; Kihal, M. Antimicrobial activity of lactic acid bacteria and the spectrum of their biopeptides against spoiling germs in foods. Braz. Arch. Biol. Technol. 2012, 55, 435–444. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Zehra, S.A.; Javed, S.; Nadeem, S.G.; Hakim, S.T. Lactic acid bacteria from fresh fruits and vegetables as biocontrol agent of foodborne bacterial pathogens. RADS J. Biol. Res. Appl. Sci. 2014, 5, 36–45. [Google Scholar]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikien, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Cálix-Lara, T.F.; Rajendran, M.; Talcott, S.T.; Smith, S.B.; Mille, R.K.; Castillo, A.; Sturino, J.M.; Taylor, T.M. Inhibition of Escherichia coli O157: H7 and Salmonella enterica on spinach and identification of antimicrobial substances produced by a commercial lactic acid bacteria food safety intervention. Food Microbiol. 2014, 38, 192–200. [Google Scholar] [CrossRef]
- Malik, D.K.; Bhatia, D.; Nimbriya, A.; Kumar, S. Lactic acid bacteria and bacteriocin: A Review. J. Pharm. Res. 2012, 5, 2510–2513. [Google Scholar]
- Stefanis, C.; Mantzourani, I.; Plessas, S.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Kandylis, P.; Varzakas, T. Reviewing classical and molecular techniques regarding profiling of probiotic character of microorganisms. Curr. Res. Nutr. Food Sci. 2016, 4, 27–47. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of milk type on the microbiological, physicochemical and sensory characteristics of probiotic fermented milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO, Food and Nutritional Paper No. 85; FAO: Rome, Italy, 2006; pp. 1–50. [Google Scholar]
- Ayala, F.R.; Bauman, C.; Cogliati, S.; Leñini, C.; Bartolini, M.; Grau, R. Microbial flora, probiotics, Bacillus subtilis and the search for a long and healthy human longevity. Microbial. Cell. 2017, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020, 140, 103931. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Blagojev, N.; Škrinjar, M.; Vesković-Moračanin, S.; Šošo, V. Control of mould growth and mycotoxin production by lactic acid bacteria metabolites. Rom. Biotechnol. Lett. 2012, 17, 7219–7226. [Google Scholar]
- Sathe, S.J.; Nawani, N.N.; Dhakephalkar, P.K.; Kapadnis, B.P. Antifungal lactic acid bacteria with potential to prolong shelf life of fresh vegetables. J. Appl. Microbiol. 2007, 103, 2622–2628. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8060952
