Geopolymer technology (GCs) refer to a class of alumino-silicate cementitious materials resulting from an inorganic polycondensation reaction (named “geopolymerization”) between solid alumino-silicate precursors and highly concentrated aqueous alkali hydroxide or silicate solution such as sodium hydroxide (NaOH), potassium hydroxide (KOH), sodium silicate (Na2SiO3), or potassium silicate (K2SiO3).
- geopolymer technology
- carbon dioxide emission
- alumino-silicate sources
- rheology
- mechanical properties
- durability
- microstructure
- thermo-acoustic properties
- additive manufacturing
- civil applications
1. Introduction
Portland cement (PC) production is responsible for 5–8% of all man-made carbon-based greenhouse emissions across the globe, corresponding to 0.6–0.8 kg of CO2 generated for every kg manufactured [1][2]. The entire cement production chain (Figure 1), including the extraction of raw materials from the mine and their preparation, the calcination process in the clinker furnaces, and the thermal emission related to the process selectively contributes to the release of CO2.
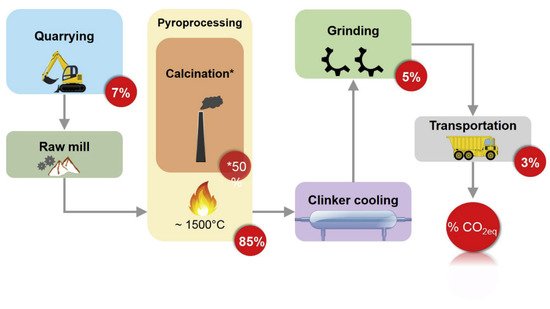
Figure 1. Diagram on PC manufacturing chain and the CO2 emission rate (in percentage) associated with each stage (reproduced with permission from Maddalena, R. et al., Journal of Cleaner Production; Elsevier, 2018).
Geopolymer materials are zero-PC binders formed through a polycondensation reaction of alumino-silicate sources, derived from waste by-products, in alkaline solutions. The process, commonly defined as “geopolymerization”, produces a three-dimensional (3D) inorganic network with an amorphous/semi-crystalline microstructure. Unlike the ordinary PC, in which calcium silicate hydrates (C-S-H) gel is the main binding compound, GC utilizes the polycondensation of Silica (SiO2) and alumina (Al2O3) sources and a high-alkali environment to obtain structural strength [3][4]. Recent studies confirm the remarkable functionality of geopolymer technology in terms of eco-sustainability and engineering properties. Compared with conventional PC, the superior eco-efficiency of GCs is mainly attributable to the reduced CO2 footprint, the employment of low-temperature processing, and the use of by-product materials as precursors, which prevents the accumulation of wastes in landfills [5].
2. Geopolymer Cements (GCs): Chemistry, Raw Materials, and Products
Davidovits [6] provided the main contribution to the discovery and scientific research of geopolymer materials. In the 1980s, he developed the first inorganic polymer by geopolymerization of natural minerals containing silicon (Si) and aluminum (Al), such as clay, slag, fly ash, pozzolan, and alkaline activator below 160 °C [7].
The chemistry of geopolymerization mechanism involves the following steps:
-
(1)
-
Alkali activation. An alkaline activator is necessary for the dissolution of Si and Al from the inorganic precursor as well as for the catalysis of the condensation reaction. The reaction of alumino-silicate oxides (Si2O5, Al2O2) in strongly alkaline solution results in a breakdown of Si-O-Si bonds with the subsequent penetration of Al atoms in the original Si-O-Si structure. The resulting alumino-silicate oxide gels, based on Si-O-Al block, are the geopolymer precursor of the polycondensation reaction. The dissolution/hydrolysis reaction is reported in Figure 2.
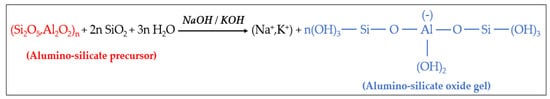
Figure 2. Dissolution and hydrolysis reaction of the alumino-silicate precursor.
-
(2)
-
Polycondensation in Geopolymer network. The alumino-silicate gel phase is a highly reactive product. Under alkaline condition, substantially fast chemical reactions occur, forming a rigid 3D polymeric and ring framework of Si-O-Al bonds (Figure 3). The proper completion of the geopolymerization process and the conferment of adequate mechanical strength properties to the material require heat curing treatment at a thermal range between 25 and 90 °C [8]. The water released by polycondensation is normally consumed during the dissolution process.
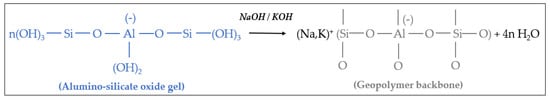
Figure 3. Polycondensation of hydrolyzed aluminate and silicate species and formation of 3D geopolymer network.
Geopolymer networks are polysialate structures, where sialate indicates a Si-O-Al building unit. The Si/Al atomic ratio significantly determines the final reticular structure and degree of crystallinity of the resulted geopolymer binders. Depending on the Si/Al molar proportion, three silicon-oxo-aluminate tetrahedral structures develop (Figure 4): (Si-O-Al-O)-type polysialate, (Si-O-Al-O-Si-O)-type polysialate-siloxo, and (Si-O-Al-O-Si-O-Si-O)-type polysialate disiloxo [7].
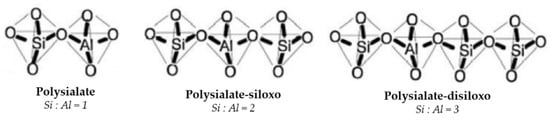
Figure 4. Chemical structures of polysialates.
In agreement with Duxson et al. [9], together with processing conditions, the selection of raw materials is critical in determining the rheological, chemical, physical, and mechanical properties of geopolymer products. GCs can be classified according to the type of alumino-silicate precursor in metakaolin-based geopolymer cements (MGCs), fly ash-based geopolymer cements (FGCs), natural minerals-based geopolymer cements (NGCs), and hybrid geopolymer cements (HGCs).
2.1. Metakaolin-Based Geopolymer Cements (MGCs)
Metakaolin (MK), or calcinated kaolin, is a ceramic powder based on calcined clay that is formed during the calcination process at temperatures between 500 and 800 °C. Thanks to its pozzolanic properties and the reduced thermal energy requirements for its production (80–90% less CO2 emission than PC), it is generally used to replace traditional cementitious binders to obtain more eco-sustainable building materials. Compared to other alternative binders applied in cement manufacturing (fly ash or blast furnace slags), MK is not a by-product resulting from an industrial process but is obtained in specific calcination conditions [10]. The high specific surface area and plate-shaped texture negatively affect the workability of geopolymer compounds, increasing the processing complexity and the water demand [11]. The last aspect promotes the strong tendency to a large degree of drying shrinkage and cracking. Although MK is a non-renewable resource, the higher concentration of reactive material and purity than other alumino-silicate precursors is beneficial aspects to obtain high-strength and low permeability properties in geopolymer compounds [12].
2.2. Fly Ash-Based Geopolymer Cements (FGCs)
Low-calcium FA (designated as “F” class) possess pozzolanic properties and was extensively used as a supplementary cementitious material in the PC manufacturing process to minimize the greenhouse emissions, which occur using traditional pozzolanic aggregates. As reported by Vargas and Halog [13], the employment of industrial waste or by-products for clinker replacement can reduce CO2 emissions up to 12% when 10% of these secondary raw materials is incorporated in the cementitious mix. Reduction in heat of hydration, increase in workability, and improvement of durability to chemical attacks are other engineering benefits obtainable by using FA in blended cements [14][15].
As shown in Table 1, the high percentage of SiO2 and Al2O3, makes fly ashes suitable for GCs manufacturing. Variations in the compositional proportion are mainly attributable to the type of raw coal-based materials (anthracite coal or lignite) involved in the process. Compared to the MK precursor, FA-based geopolymer does not require high-temperature processing and a high level of energy consumption [16]. Although FGCs exhibit good mechanical strength and durability, the limiting factor in the use of FA as geopolymer precursors is the low reactivity. The incomplete dissolution of FA leads to slowing the setting and strength development [17].
Table 1. Geopolymer concrete samples investigated by Kashani et al.: precursors proportions, particle size, and dry packing density (adapted from [18]).
| Formulation | FA(%) | GBFS (%) | Micro-FA (%) |
d (μm) | Dry Packing Density (0–1) |
|---|---|---|---|---|---|
| M 1 | 5 | 25 | 10 | 33.1 | 0.483 |
| M 2 | 10 | 20 | 10 | 37.7 | 0.505 |
| M 3 | 15 | 15 | 10 | 42.8 | 0.517 |
| M 4 | 15 | 20 | 5 | 43.5 | 0.515 |
| M 5 | 20 | 15 | 5 | 48.6 | 0.528 |
The micromorphology difference between MGC and FGC is evident. MGC shows a uniform layer-like structure, overall free of voids or cracks (Figure 5a). Comparatively, heterogeneous structure and hollow cavities can be observed in FGC (Figure 5b). Porous microstructure results from the partial alkaline dissolution of FAs, which releases spherical pore in the matrix. Unreacted particles can be located inside these cavities.
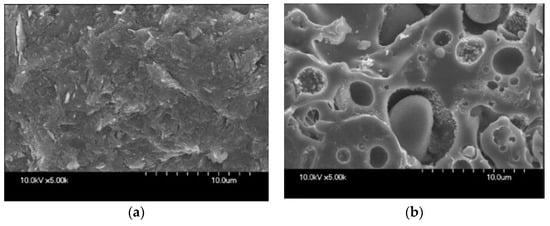
Figure 5. SEM micrographs of MGC (a) and FGC (b) microstructures (reproduced with permission from Kong et al., Cement and Concrete Research; Elsevier, 2007).
2.3. Natural Minerals-Based Geopolymer Cements (NCs)
The synthesis of NGCs includes a variety of natural virgin raw materials such as alumino-silicate precursors. Pumice-type natural pozzolana, natural zeolite, volcanic ash, and mining waste are the main examples of natural materials used as a pozzolanic source for geopolymer preparation. In the geopolymer technology context, several studies have revealed the influence of pumice precursors in enhancing the lightweight and absorption resistance of the material [19][20]. The typical micro-porous morphology of zeolite can extend the functionality of geopolymer materials in terms of purification of contaminated water, encapsulation of waste material, CO2 adsorption, and stabilization of heavy metals. Extensive studies on the suitability of volcanic ash as a feedstock for GC synthesis were conducted by Djobo et al. [21][22]. The improved durability performances (wet–dry conditions and sulfuric attack) are the most attractive effect highlighted by the results. High acid resistance is attributable to the formation of sodium (Na)-rich gel after geopolymerization, which neutralizes sulfuric acid mitigating its destructive effects on the geopolymer structure.
2.4. Hybrid Geopolymer Cements (HGCs)
“Hybrids” refers to geopolymer binders obtained from a blend of pozzolanic precursors having complementary properties. The scientific literature provides numerous examples of GCs based on alkali silicate-activated blends. Bernal et al. [23] investigated the influence of including granulated blast furnace slags (GBFSs) in MK-based geopolymers in terms of physical–mechanical performances. GBFS, a by-product of the steel manufacturing industry, essentially consists of SiO2, Al2O3, calcium oxide (CaO), and magnesium oxide (MgO). The alkaline activation leads to the dissolution of Ca2+ ions, which implies the development of a microstructure rich in stable and high-density hydrate Ca-silicate (C-S-H) phases, resulting in higher mechanical strength than the unblended system (about 60% increase). Under alkaline conditions, the C-S-H gel changes in Na-Ca-silicate hydrate gel (C-N-S-H phase), which confers high durability to GC when exposed to CO2-rich environments. Highly condensed binder gel and the low content of chemically bonded water in the alkali-activated products promote high stability under high-temperature expositions, providing an attractive technological alternative to traditional cementitious materials in building applications where high thermal conditions occur.
3. Recent Research Findings of Geopolymer-Based Concrete Properties
Similarly to ordinary Portland-based mixes, geopolymer concrete/mortars consist of the proper combination between mineral aggregates (sand, gravel, stone), chemical admixtures, and geo-cementitious binders (Figure 7).
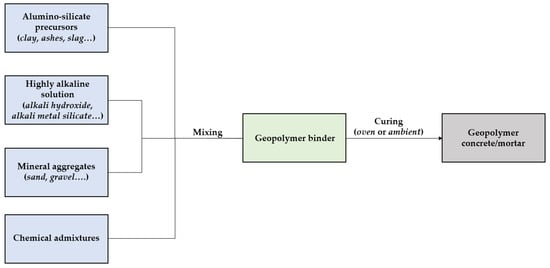
Figure 7. Production of geopolymer concrete. A descriptive model.
3.1. Rheological Properties
The following diagram (Figure 8) illustrates the influence of rheological parameters (YS and PV) on the performance and quality of fresh cementitious conglomerate.
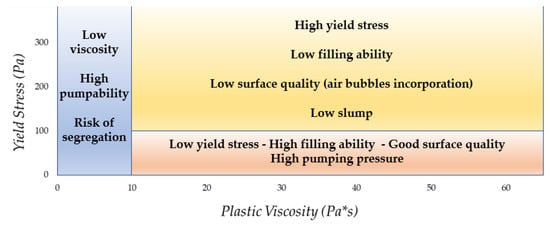
Figure 8. Summary of the impact of rheological parameters on concrete performance (adapted from [24]).
3.2. Microstructural Properties
The pore architecture of hardened geopolymeric compounds derives from the complex interaction of intertwined factors, such as size gradation of alumino-silicate precursors, geopolymerization reaction rate, solvent type, mixing proportions, and curing conditions. Generally, in GC matrices, it is possible to detect the coexistence of different void systems [25]: macro-porosity (>50 nm), meso-porosity (from 2 nm to 50 nm), and micro-porosity (<2 mm). Macropores are more interconnected than meso and micropores.
Mesopores represent the typical pores between geopolymer phases. Micropores represent the voids structure of the geopolymer gel network The microstructure of the GCs is highly sensitive to process parameters and the chemical–physical characteristics of the raw materials; therefore, the pore size distribution can shift from macro-porosity to meso or micro-porosity as a function of process variables.
3.3. Mechanical Properties
The complex relationship between the mechanical behavior of geopolymer composites and synthesis parameters, including the reactivity of aluminosilicate precursors, Si/Al ratio, the concentration of alkali activator solution, curing regime, water-to-geopolymer binder ratio (amount of water present in solution and extra water added in the mix + solid precursor and mass of alkali reagents), and type of mineral aggregates is well presented in most research studies [19][18][26][27][28][29]. The influence of these factors on the strength optimization of geocement-based concrete/mortars is summarized in Figure 12.
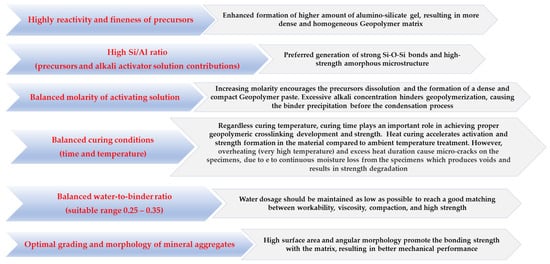
Figure 12. How the synthesis parameters optimize the strength properties of GC composites.
3.4. Durability Properties
The durability of building materials strongly affects the service life of structural components. High durability means protecting the structural reinforcements (e.g., steel armor) from corrosion and reducing the deterioration of the material under hostile chemical attacks or fires. Recent research works on durability performances demonstrate that geopolymer concrete can be considered a possible alternative material to ordinary Portland aggregate.
3.5. Thermal and Acoustic Properties
Pasupathy et al. [30] developed an ultra-lightweight FA-GGBS geopolymer foamed concrete (<600 kg/m3) using porous lightweight aggregates, i.e., expanded perlite (EP), and premade foam activator (Na-dodecyl sulfate solution). The addition of EP increased fine air voids in the geopolymer matrix (Figure 15), resulting in an advantageous improvement in mechanical and thermal properties.
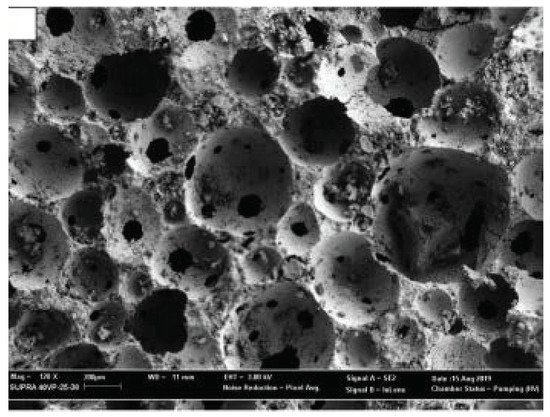
Figure 15. Examples of geopolymer compounds with improved thermal functionality: FGC microstructure (reproduced with permission from Pasupathy et al., Construction and Building Materials; Elsevier, 2020).
An alternative approach to FGCs is the functionalization of geopolymer compounds with highly thermo-insulating waste products. Ahmed et al. [31] investigated the effect of different additions of clay brick waste powder (CBP) and aggregates (CBAs) on the thermal conductivity of MK-based geopolymer concrete. The heat conductivity of the reference mix (1.53 W/m·K) declined up to 46% and 54% for mixes with 30% of CBP and CBAs, respectively.
In terms of acoustic performance, some research studies demonstrated the high sound absorption efficiency of FGCs. The cellular microstructure promotes the friction-energy loss and the dissipation into the heat of the acoustic waves during the continuous collision with the micro holes. Regarding pore structure, only open porosity is beneficial to absorption behavior, as the poro-acoustic interaction into the cement medium is encouraged, and sound reflection is minimized. Leiva et al. [32] reported acoustic absorption coefficients (α) over 0.40 at middle-high frequencies (1000–2000 Hz) and high frequency (3500–4000 Hz) in a porous FA-geopolymer concrete (22% open porosity) based on Paval (a solid waste steam from the Al industry) as the foaming agent.
4. Conclusions
Significant advances were also made in terms of technological innovation and applicability. One of the main interesting goals achieved concerns the possibility of modifying geopolymer mixes for advanced AM processes, opening up to a new design approach, and engineer optimization in the construction industry. In this regard, future work must be done to better understand the possibility of using 3D printing technology to develop functional geopolymer-based applications in the building–architectural fields.
Although more progress has been made regarding the diffusion of these building materials in the civil sector (multi-cooperation scientific projects and extensive development of start-up companies), their use would still seem weak. The main limiting factors, on which more investigation will be needed, are summarized in the following points:
-
The high sensitivity of the geopolymerization process to environmental factors and synthesis parameters requires skilled and highly trained labor to obtain materials of suitable quality. The instability in the chemical composition of precursors can be another severely limiting factor.
-
High costs and toxicity of activating alkaline solutions. In this regard, the study of more eco-friendly and cheap activators could be a possible way of research to optimize the geopolymer technology.
-
Long-term availability of raw materials. The stringent environmental regulations adopted in many industrialized countries on the use of renewable resources as primary energy supplies have led to a slight decline in many power plants, from which geopolymeric raw materials are extracted (for example, coal-fired power stations for FA supply). If this trend continues, it may further affect the diffusion of GC as a replacement of ordinary PC. However, in accordance with current production rates, availability, and costs, possible replacements of PC concrete with geopolymer aggregates of at least 75% are feasible [33].
This entry is adapted from the peer-reviewed paper 10.3390/jcs5040109
References
- BBC. Available online: (accessed on 17 March 2021).
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130.
- Van Chanh, N.; Trung, B.D.; Van Tuan, D. Recent research geopolymer concrete. In Proceedings of the 3rd ACF International Conference-ACF/VCA, Ho Chi Minh City, Vietnam, 11–13 November 2008; Volume 18, pp. 235–241.
- Nuaklong, P.; Jongvivatsakul, P.; Pothisiri, T.; Sata, V.; Chindaprasirt, P. Influence of rice husk ash on mechanical properties and fire resistance of recycled aggregate high-calcium fly ash geopolymer concrete. J. Clean. Prod. 2020, 252, 119797.
- Ahdaya, M.; Imqam, A. Investigating geopolymer cement performance in presence of water based drilling fluid. J. Pet. Sci. Eng. 2019, 176, 934–942.
- Davidovits, J. Geopolymer cement. A Rev. Geopolymer Inst. Tech. Pap. 2013, 21, 1–11.
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746.
- Kani, E.N.; Allahverdi, A. Effects of curing time and temperature on strength development of inorganic polymeric binder based on natural pozzolan. J. Mater. Sci. 2009, 44, 3088–3097.
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933.
- Calcestruzzo&Prefabbricazione International. Available online: (accessed on 2 January 2021).
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90.
- Zhang, Z.; Wang, H.; Zhu, Y.; Reid, A.; Provis, J.L.; Bullen, F. Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl. Clay Sci. 2014, 88, 194–201.
- Vargas, J.; Halog, A. Effective carbon emission reductions from using upgraded fly ash in the cement industry. J. Clean. Prod. 2015, 103, 948–959.
- Arnon, C.; Thanongsak, N. Thermal analysis and microstructure of Portland cement-fly ash-silica fume pastes. J. Therm. Anal. Calorim. 2009, 99, 487–493.
- Nochaiya, T.; Wongkeo, W.; Chaipanich, A. Utilization of fly ash with silica fume and properties of Portland cement–fly ash–silica fume concrete. Fuel 2010, 89, 768–774.
- Kong, D.L.; Sanjayan, J.G.L.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589.
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615.
- Kashani, A.; Ngo, T.D.; Mendis, P. The effects of precursors on rheology and self-compactness of geopolymer concrete. Mag. Concr. Res. 2019, 71, 557–566.
- Ekmen, Ş.; Mermerdaş, K.; Algın, Z. Effect of oxide composition and ingredient proportions on the rheological and mechanical properties of geopolymer mortar incorporating pumice aggregate. J. Build. Eng. 2020, 101893.
- Binici, H. Engineering properties of geopolymer incorporating slag, fly ash, silica sand and pumice. Adv. Civ. Env. Eng. 2013, 1, 108–123.
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Mechanical activation of volcanic ash for geopolymer synthesis: Effect on reaction kinetics, gel characteristics, physical and mechanical properties. RSC Adv. 2016, 6, 39106–39117.
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Mechanical properties and durability of volcanic ash based geopolymer mortars. Constr. Build. Mater. 2016, 124, 606–614.
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486.
- Ferraris, C.F.; Billberg, P.; Ferron, R.; Feys, D.; Hu, J.; Kawashima, S.; Koehler, E.; Sonebi, M.; Tanesi, J.; Tregger, N. Role of rheology in achieving successful concrete performance. ACI Commun. 2017, 238, 43–51.
- Gunasekara, C.M. Influence of Properties of Fly Ash from Different Sources on the Mix Design and Performance of Geopolymer Concrete. Ph.D. Thesis, School of Engineering College of Science, Engineering and Health RMIT University Melbourne, Melbourne, Australia, July 2016.
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment–A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728.
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M. Thermo-mechanical characteristics of geopolymer mortar. Constr. Build. Mater. 2019, 213, 100–108.
- Hu, Y.; Tang, Z.; Li, W.; Li, Y.; Tam, V.W. Physical-mechanical properties of fly ash/GGBFS geopolymer composites with recycled aggregates. Constr. Build. Mater. 2019, 226, 139–151.
- Mermerdaş, K.; Manguri, S.; Nassani, D.E.; Oleiwi, S.M. Effect of aggregate properties on the mechanical and absorption characteristics of geopolymer mortar. Eng. Sci. Technol. Int. J. 2017, 20, 1642–1652.
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Enhancing the mechanical and thermal properties of aerated geopolymer concrete using porous lightweight aggregates. Constr. Build. Mater. 2020, 264, 120713.
- Ahmed, M.F.; Khalil, W.I.; Frayyeh, Q.J. Thermal Insulation Enhancement of Metakaolin-Based Geopolymer Concrete Using Waste Clay Brick. In Proceedings of the 4th International Conference on Manufacturing Technologies (ICMT), Seattle, WA, USA, 17–19 January 2020.
- Leiva, C.M.; Luna-Galiano, Y.; Arenas, C.; Alonso-Fariñas, B.; Fernández-Pereira, C. A porous geopolymer based on aluminum-waste with acoustic properties. Waste Manag. 2019, 95, 504–512.
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477.
