The use of nitrogen by plants involves several steps, including uptake, assimilation, translocation, and different forms of recycling and remobilization processes, all of them of crucial importance in terms of nitrogen utilization efficiency. Different processes exist in plants, which give rise to the production of endogenous sources of ammonium which have to be efficiently re-assimilated by secondary ammonium assimilation. These processes include photorespiration, the biosynthesis of phenylpropanoids, as well as ureide, nucleotide and amino acid catabolism [
1]. Phenylpropanoid metabolism represents an important metabolic pathway from which originates a wide number of secondary metabolites derived from phenylalanine or tyrosine, including monolignols, flavonoids and isoflavonoids, various phenolic acids, and stilbenes [
2]. It is well known that secondary metabolites are crucial molecules in plant life, as protective agents against environmental factors (e.g., oxidative stress, pathogens, etc.) as well as elements favoring reproduction [
3,
4,
5,
6]. In particular, it is well established that phenylpropanoid-derived compounds have roles in plant growth and development, and in the defense against biotic and abiotic stress [
2]. The phenylpropanoid pathway has different branches that lead to different families of compounds, such as chalcones, flavones, flavonols, flavanones, isoflavonoids, and anthocyanins, among others [
7]. The structure, composition and biological activity of flavonoids have been frequently analyzed (see [
7,
8,
9,
10] for an overview, and references therein).
The second most important family of crop plants for humans, after Poaceae, are Fabaceae because they provide sources of food, feed for livestock and raw materials for industry [
11]. Legumes are crucial plants in sustainable agriculture because they are able to fix atmospheric dinitrogen in a symbiotic association with rhizobial species. In addition, legumes produce a high diversity of secondary metabolites which serve as defense compounds against herbivores and microbes, but also as signal compounds to attract pollinating and fruit-dispersing animals. As nitrogen-fixing organisms, legumes produce more nitrogen containing secondary metabolites than other plant families [
12]. In particular, flavonoids and isoflavonoids, which are compounds lacking nitrogen in their structures, are postulated to play pivotal roles in the adaptation of legumes to their biological environments both as defensive compounds (phytoalexins) and as chemical signals in symbiotic nitrogen fixation with rhizobia [
13]. A primary function of flavonoids in legume–rhizobia symbiosis is to induce transcription of the genes involved in the biosynthesis of Nod factors. These factors are rhizobial signaling molecules perceived by the plant to allow symbiotic infection of the root. Many legumes produce specific flavonoids that only induce Nod factor production in compatible rhizobia, and therefore act as important determinants of host range [
14]. Despite a wealth of evidence on legume flavonoids, relatively few have proven roles in rhizobial infection. The molecular details of how flavonoid production in plants is regulated during nodulation have not yet been clarified, but nitrogen availability has been shown to play a role [
15]. The role of flavonoids and isoflavonoids in plant symbiosis is not limited to nitrogen-fixing bacteria since these compounds also play several roles in the symbiosis with mutualistic fungi. During the establishment of fungal symbiosis, these compounds can stimulate spore germination, hyphal branching and growth, root colonization, and arbuscule formation inside the root [
16]. In later stages of symbiosis, flavonoids may be involved in the autoregulation of mycorrhization [
17]. In the case of soybean, a specific isoflavonoid rather than a flavonoid can stimulate hyphal growth [
18]. These effects often are host-specific, much like in the case of plant–rhizobial symbiosis. In fact, autoregulation of nodulation and autoregulation of mycorrhizae, the two negative feedback loops that control the formation of rhizobial and mycorrhizal symbioses, may share common elements [
19]. However, the inhibitory effects of some plant flavonoids on fungal symbiosis have also been reported, both in plants that are host for mycorrhizal fungi and in non-host plants ([
16], and references therein). Flavonoids can also accumulate in the early stages of plant–fungi interaction as a defense response; however, once the symbiosis has been established, the fungal symbiont may use the flavonoids as carbon source [
20]. In addition, because legumes are a significant source of food and forage, the effects of leguminous flavonoids and isoflavonoids on human and animal health are being studied intensively [
13,
21]. In particular, excellent reviews describe exhaustively the different isoflavonoids compounds found in legume plants [
22,
23,
24].
In this paper, we will summarize recent progress made in the characterization of flavonoid and isoflavonoid biosynthetic pathways in legume plants with a particular focus on the model legume Lotus japonicus, and the impact that these studies may have to improve cultivated legumes of great agronomic importance such as soybean (Glycine max).
Flavonoid and Isoflavonoid Biosynthetic Pathways in Lotus
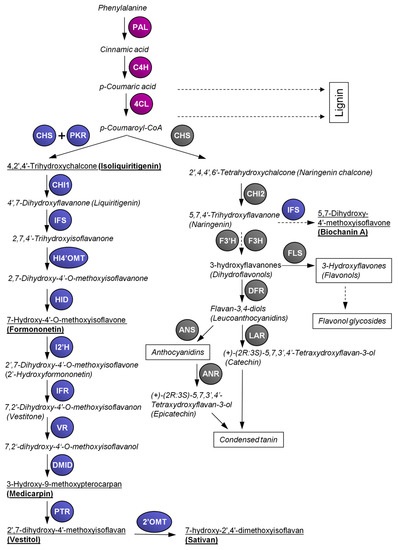
The enzyme chalcone synthase (CHS) is involved in the biosynthesis of the precursor molecules for both flavonoids and isoflavonoid biosynthesis. CHS is a member of the type III polyketide synthase family that catalyzes the conjugation of three acetate units from malonyl-CoA to a
p-coumaroyl-CoA starter molecule derived from phenylalanine via the general phenylpropanoid pathway (). In the same active site, additional aromatic “A” cycle of flavonoids is built via the intramolecular cyclisation [
39]. The product of such reaction is 2’,4,4’,6’-tetrahydroxychalcone (naringenin chalcone), later changing to 5,7,4’-trihydroxyflavanone (naringenin) by building of the “C” heterocycle catalyzed by chalcone isomerase (CHI) that serves as a precursor for the other flavonoids [
40]. In some species of the family Fabaceae, isoflavonoids, such as genistein, biochanin A or others, are produced from naringenin [
41]. However, most of the isoflavonoids are synthesized via isoliquiritigenin that is produced by the coupled catalytic action of CHS and chalcone reductase (CHR; also called polyketide reductase, PKR, see below).
Figure 1. Overview of the flavonoid and isoflavonoid pathways in
Lotus japonicus. 4CL, 4-coumarate:CoA ligase; 2’OMT, 2’-O-methyltransferase; I2’H, isoflavone-2’-hydroxylase; ANR, anthocyanidin reductase; ANS, anthocyanidin synthase; C4H, cinnamic acid 4-hydroxylase; CHI, chalcone isomerase; DMID, 7,2’-dihydroxy-4’-O-methoxyisoflavanol dehydratase (syn. pterocarpan synthase); CHS, chalcone synthase; DFR, dihydroflavonol 4-reductase; F3H, flavanone 3-hydroxylase; F3’H, flavanone 3’-hydroxylase; FLS, flavonol synthase; HID, 2-hydroxyisoflavanone dehydratase; HI4’OMT, 2-hydroxyisoflavanone 4’-
O-methyltransferase; IFR, isoflavone reductase; IFS, 2-hydroxyisoflavanone synthase; LAR, leucoanthocyanidin reductase; PAL, phenylalanine ammonia lyase; PKR, polyketide reductase (syn. chalcone reductase); PTR, pterocarpan reductase; VR, vestitone reductase. Purple color: enzymes of general phenylpropanoid pathway; grey color: enzymes of flavonoid pathway; blue color: enzymes of isoflavonoid pathway. Dashed arrows represent multiple biosynthetic steps. Trivial names of compounds are presented if they are commonly used; the others are presented by their semi-systematic names. Semi-systematic names and chemical structures of the referred flavonoids and isoflavonoids are attached online in
Table S1. The names underlined in bold highlight most abundant isoflavonoids found in
L. japonicus according to our data [
42].
Whereas in
Arabidopsis thaliana only one single gene for CHS is known, in other species several
CHS genes were found (e.g., two in cacao, four in wild strawberry, five in apple, six in poplar), which is especially true for legumes [
43,
44]. In
L. japonicus 13–14
CHS genes were found, 15 in
Glycine max and 17 in
Medicago truncatula [
43]. The higher number of
CHS genes in legumes is likely related to the presence of the isoflavonoid pathway in that family. In
L. japonicus,
CHS6 (called
LjCHS1 in [
45]) could represent the non-leguminous type of chalcone synthase; on the other hand, in soybean,
GmCHS6,
GmCHS7 and
GmCHS8 seem more related to isoflavonoid production [
46,
47].
GmCHS7 and
GmCHS8 show strong homology with
LjCHS5 (Lj1g3v2626200.1),
LjCHS8 (Lj0g3v0129339.1)
LjCHS9 (Lj2g3v2124320.1) and
LjCHS11 (Lj2g3v2124320.2), whereas
GmCHS6 is homologous to
LjCHS12 (Lj4g3v2574990.1). However,
Lotus isoflavonoids are produced mainly via isoliquiritigenin, the daidzein and genistein (and their derivates) found in soybean are produced from isoliquiritigenin and naringenin chalcone, respectively [
41,
48] (). Therefore, the regulation pattern of chalcone synthases in soybean might be more complex.
The flavonoid biosynthetic pathway producing flavonols, anthocyanidins and proanthocyanidins (condensed tannins) in
L. japonicus are described in .
F3H,
F3’H and
FLS genes have not been studied in detail to date—five
DFR genes were described in a cluster on chromosome 5 by [
49] and different specificities of DFR isozymes in the substrate hydroxylation patterns have been reported. The proanthocyanidins (both epicatechin and catechin type) are biosynthesized from dihydroflavonols by the action of anthocyanidin reductase (ANR) and leucoanthocyanidin 4-reductase (LAR), two gene encodings for enzymes committed to epicatechin and catechin biosynthesis, respectively, that were identified in
L. corniculatus [
50].
Higher plants share a common core flavonoid pathway, although different species frequently develop specific branches as an adaptation to diverse environmental conditions. For example,
A. thaliana accumulates mainly flavonols (kaempferol, quercetin and isorhamnetin glycosides) in all tissues, and anthocyanidins and epicatechin types of proanthocyanidins in the seed coat under stress conditions [
51]. A rising number of studies report protein–protein interactions of flavonoid biosynthetic enzymes providing evidence for weakly bound complexes called “metabolons” which are co-localized at the endoplasmic reticulum (ER) [
52,
53,
54]. The interaction of the enzymes in the system likely allows better connection of reaction intermediates with subsequent enzymes and prevents their loss by diffusion or unfavorable cell equilibrium. Such protein–protein interactions were found for CHS, flavanone 3-hydroxylase (F3H), dihydroxyflavonol 4-reductase (DFR), anthocyanidin synthase (ANS) and also CHI or CHI-like protein (with a putative role as fatty-acid binding protein) [
55], so the proposed model of metabolon comprises the enzymes necessary for formation of anthocyanidins [
56]. On the other hand, there is still lack of evidence of interaction with flavanone 3’–hydroxylase (F3’H) [
57]. Proanthocyanidins are produced by action of ANR, LAR and polyphenol oxidase (LAC15) resulting in the oligo-and polymers of the flavan-3-ol units. Substrate channeling between DFR and LAR was described using molecular modeling and predicted the functional significance of metabolon formation during synthesis [
58]. Proanthocyanidins are produced both in shoots and roots of
Lotus sp. However, significant differences in their accumulation may occur among different species, but also within different populations of the same species. Whereas in
L. japonicus (and some other species) they are usually present in almost undetectable amounts, the closely related tetraploid forage species
L. corniculatus may accumulate proanthocyanidins in considerable levels [
59,
60]. The highest proanthocyanidin levels were found in
L. unifoliolatus (syn.
L. americanus) and
L. uliginosus (syn.
L. pedunculatus) [
59,
61].
The key enzyme for flavonol formation is flavonol synthase (FLS) using dihydroflavonol substrates.
L. japonicus is a plant that accumulates flavonol kaempferol glycosides in considerable amounts, especially kaempferol-3,7-dirhamnoside. Quercetin glycosides are present at lower levels but increase under some abiotic stress conditions [
62,
63]. Moreover, a considerable amount of gossypetine glycosides occurs in flowers and a small amount of isorhamnetine can be detected in stems [
64]. Only the minor methylation on 3’ position of quercetine is present in
L. japonicus, whereas the methylation at position 8 was described only in
L. corniculatus [
65], leading to presence of sexangularetin and corniculatusin in that species [
66].
The production of isoliquiritigenin, the starting point of the second branch of the biosynthetic pathway, is related to the activity of CHR (also called polyketide reductase, PKR), only identified in papilionoid legumes (like
Glycine max,
Medicago sativa,
Glycyrrhiza echinata,
Glycyrrhiza glabra). Five genes and 1 pseudogene are present in the
L. japonicus genome [
35]. CHR acts in a coupled catalytic action with CHS [
45]. Furthermore, two types of CHI genes are present.
LjCHI2 is highly homologous to non-legumes (also referred as type I), whereas
LjCHI1,
LjCHI3 and
LjCHI4 are legume-specific type II, also occurring in
Medicago sativa,
Phaseolus vulgaris,
Pisum sativum and
Pueraria lobata [
67] (). The legume-specific type II evolved to produce 5-deoxy(iso)flavonoids from 6’deoxychalcone (isoliquiritigenin) along with the establishment of the Fabaceae.
The protein–protein interaction of key enzymes of isoflavonoid pathway (CHS, CHR, CHI and IFS) that are associated with ER via cytochrome P450 has been recently demonstrated in soybean [
68] as well as with the three enzymes of general phenylpropanoid pathway (PAL, C4H, 4CL) and with the last enzyme of the shikimate pathway, arogenate dehydratase (ADT), the enzyme converting arogenate to phenylalanine [
69]. The enzyme complex may be associated with the ER membrane at the plastid-associated membrane sites, allowing the flux of intermediates from shikimate pathway occurring in plastids toward daidzein or glycitein isoflavones present in soybean [
69,
70].
Isoflavone synthase (IFS; 2-hydroxyisoflavanone synthase) is a membrane-associated enzyme belonging to the CYP93C subfamily of cytochrome P450 monooxygenases that constructs the isoflavonoid skeleton from 4’,7-dihydroxyflavanone substrate (liquiritigenin) by an unusual aryl migration reaction. At a lower rate, IFS may convert naringenin in several legume species, such as soybean [
41]. IFS has been identified almost exclusively in legumes, with
Beta vulgaris being the only known exception [
71,
72]. Among 273 putative P450 genes in
A. thaliana genome, none of them has isoflavone synthesizing activity [
73]. At least two functional genes of IFS (
IFS1 and
IFS2) and one pseudogene are present in the
L. japonicus genome [
45].
L. japonicus IFS likely has a strong preference for liquiritigenin, although a small amount of biochanin A detected in plants on UV-B irradiation suggests a possibility of a minor activity using also naringenin as a substrate [
42].
The substrate specificity of 2-hydroxyisoflavanone dehydratase (HID) may differ among species. In soybean, HID accepts 2,5,7,4’-tetrahydroxyisoflavanone or 2,7,4’-trihydroxyisoflavanone as substrate, which is then de-hydrated to produce a double bond between C-2 and C-3, yielding genistein or daidzein [
23,
74]. The overexpression of HID from soybean with broad substrate specificity in
L. japonicus resulted in the production of considerable amounts of daidzein or genistein [
75]. The biosynthesis of the main isoflavonoid, vestitol, in
L. japonicus was proposed by [
45], according the previous data described in
Glycyrrhiza echinata [
74]. Firstly the 4’-O-methyltransferase (HI4‘OMT) reaction occurs, and subsequent dehydration by HID yields formononetin (), the central biosynthetic intermediate for the production of diverse isoflavonoid phytoalexins (e.g., maackiain, pisatin, medicarpin, etc.) in a number of legume species, including agronomically important ones such as pea (
Pisum sativum) or chickpea (
Cicer arietinum) [
76].
In
L. japonicus, formononetin is then converted by isoflavone-2’-hydroxylase (I2’H) to 2’,7-dihydroxy-4’-O-methoxyisoflavone and subsequently to vestitone by isoflavone reductase (IFR). The next step is the NADPH-dependent reduction of vestitone to 7,2’-dihydroxy-4’-O-methoxyisoflavanol, catalyzed by the vestitone reductase (VR) that is stereospecific for the (
3S)-vestitone [
77].
HI4’OMT,
HID and
I2’H were suggested to occur in single copies in the
L. japonicus genome [
78], but recently, more putative copies could be predicted at least in the case of
HID (miyakoguza.jp 3.0). The putative
L. japonicus IFR1 and
VR1,
VR2,
VR3 and
VR4 genes (four
VR genes) for vestitol accumulation were identified by sequence similarity with
Medicago sativa [
45]. Although their functional validation is still lacking, these genes are markedly upregulated after glutathione treatment [
79]. The production of medicarpin from 7,2’-dihydroxy-4’-O-methoxyisoflavanol is catalyzed by pterocarpan synthase (PTS) that was found in
L. japonicus,
Glycine max and
Glycyrrhiza echinata. This enzyme has similar biochemical properties as previously reported DMI-dehydratase in
Cicer arientinum,
G. max and
Medicago sativa. This raises the question of whether the product of the
LjPTS1 gene corresponds to the enzyme described above, but the evidence available at present is not conclusive [
80]. The synthesis of vestitol is then catalyzed by pterocarpan reductase (PTR). Four genes were found to encode PTR, from which
PTR3 was found to be inducible by glutathione [
45]. However,
PTR1 and
PTR2 have much higher activity and enantiospecifity with (-)-medicarpin; therefore, they are considered to be responsible for vestitol production [
81]. In stress conditions like UV-B application or glutathione treatment, a remarkable accumulation of sativan was observed in
L. japonicus and
L. corniculatus [
42,
82]. Production of sativan requires the activity of a 2’-O-methyltransferase to convert vestitol to sativan. Among the type I O-methyltransferases isolated from
Medicago truncatula,
MtOMT2,
MtOMT4,
MtOMT5,
MtOMT6 and
MtOMT7 showed some vestitol methylation activity, but with a very low efficiency. Furthermore, they appeared to methylate vestitol at the positions 7 and/or 4’; any clear evidence of methylation at 2’ position of vestitol is still lacking [
83]. Vestitol is a predominant isoflavonoid produced in
L. japonicus, present in very small amount in unstressed conditions, but increases significantly at biotic [
84,
85] or abiotic stresses [
62] or after treatment with 10 mM glutathione [
78,
86]. To a lesser extent, sativan also accumulates in such conditions. Other isoflavonoids, such as formononetin and biochanin A, were raised after UV-B irradiation but their levels remained more than ten-times lower in comparison to vestitol. Accumulation of sativan and medicarpin was also detected, but in an even lower extent [
42].
Glycosylation is a major decorative modification that occurs frequently as a last step of the biosynthesis of certain flavonoids or isoflavonoids. UDP sugar residues are attached to the flavonoid core via a uridine diphosphate glycosyltransferase (UGT) [
87]. A large number of putative UGT genes have been identified in several plant species. However, only few of them were functionally characterized, mostly in
Arabidopsis thaliana [
88]. In the
L. japonicus genome, 188 putative UGT genes were identified by genome-wide searching [
89]. Tree UGT proteins of the UGT72 family enzymes (UGT72AD1, UGT72AH1 and UGT72Z2) showed narrow substrate preferences to flavonol aglycones in vitro and the overexpression of UGT72AD1 and UGT72Z2 led to increase of flavonol rhamnosides. Another two proteins, UGT72AF1 and UGT72V3, exhibited a broad activity towards flavonoids and isoflavonoids [
89]. Such a broad activity of UGTs is known also from other legumes, in particular in the case of four UGTs (GT22D, GT22E09, GT29C and GT29H) from
M. truncatula [
90] and three UGTs (UGT73F2, UGT73C20 and UGT88E19) from
G. max [
91,
92]. The UGT activity resulted to high diversity of glycosides in
L. japonicus; particularly (25) kaempferol and (12) quercetine glycosides were found mostly in flowers [
64].
Conclusions and Future Prospects
This paper summarizes recent advances made in flavonoid and isoflavonoid research in the model legume
L. japonicus. The study of the response of
L. japonicus to abiotic stress conditions led to different novel findings, such as the accumulation of new flavonols that were described for the first time in
L. japonicus leaves [
62] and of a peculiar pattern of isoflavonoid accumulation in the response of this plant to UV-B irradiation [
42]. Despite the fact that flavonoid and isoflavonoid metabolism is a very active field of research; several aspects of these pathways are far from having been completely described. Technical advances in metabolomics are enabling the discovery of a growing number of flavonoids and isoflavonoids structures. However, chemical modification of the flavonoid/isoflavonoid scaffolds, such as glycosylation and acylation, add another layer of complexity to their chemical diversity; and the reason beyond such complexity is still not completely understood. Legumes also use flavonoids or isoflavonoids in order to attract their chosen symbiont in a species-specific way. Despite the important role played by
L. japonicus in elucidating the molecular genetics of legume–rhizobia symbiosis, it is still unknown which class of phenolic compounds are used by this species in order to attract its chosen symbiont [
15]. Studies of the symbiotic capacity of specific
L. japonicus mutants impaired in specific branches of the biosynthesis of phenolic compounds, paired with metabolite profiling will be needed in order to fill this gap. The regulation of isoflavonoid metabolism is also far from being completely understood. A few negative and positive regulators have been identified in soybean, while no clear isoflavonoid regulators have been identified in
L. japonicus to date. The co-expression analysis presented in this paper identified potential candidates for isoflavonoid regulation in
L. japonicus. Future works should be aimed to the characterization of specific mutants in these genes in order to understand whether they are involved in isoflavonoid regulation, and also if they may play a role in the response to different kinds of abiotic stress. A deeper understanding of isoflavonoid regulation may also permit tackling the genetic improvement of soybean and to breed varieties with either increased or decreased isoflavonoid content, two opposite traits that can be desirable depending on the products that will be manufactured using these soya beans. Since most of the regulators identified in these species are from the MYB transcription factor family, which is composed of a very high number of genes, traditional approaches, such as searching for isoflavonoid-related QTL, may be very time consuming. Bioinformatics approaches, such as the construction and analysis of gene co-expression networks in order to find new candidate regulators, combined with validation of these genes by characterizing loss-of–function mutants, have already showed promising results. Finally, as explained in this review, in order to broaden the knowledge of flavonoid and isoflavonoid metabolism and regulation, studies that take into consideration both model species such as
L. japonicus, of easier genetic manipulation, and cultivated species of great economic importance, such as soybean, will be of paramount impact for legume flavonoid/isoflavonoid research.