Environmental pollution with alkylbenzene hydrocarbons such as toluene is a recurring phenomenon. Their toxicity and harmful effect on people and the environment drive the search for sustainable removal techniques such as bioremediation, which is based on the microbial metabolism of xenobiotic compounds.
- toluene
- biodegradation
- melanized fungi
1. Introduction
Alkylbenzenes are a subset of aromatic hydrocarbons in which one or more hydrogen atoms from benzene have been replaced by alkyl groups of different sizes. The simplest member is toluene (C6H5CH3), in which a methyl group replaces a hydrogen atom from benzene. Toluene is a common bulk chemical used worldwide as a solvent for many substances such as paints, coatings, inks, adhesives, and cleaning agents [1] and as a gasoline additive for improving octane ratings. Toluene is also useful in the benzene production process and to obtain several polymers used to manufacture synthetic materials (e.g., nylon and polyurethanes), dyes, cosmetic and pharmaceutical products, and several specialized organic chemicals [2]. Toluene is relatively soluble and volatile, so it contributes to both water and air pollution. When inhaled, it acts as a central nervous system suppressor and may be lethal after exposure for one hour at 1800 to 2000 ppm v/v [3], and it may also cause chronic toxic effects at relatively low concentrations [4][5]. The American Conference of Governmental Industrial Hygienists (ACGIH) considers 20 ppm v/v as a threshold limit value (TLV) for toluene exposure to avoid occupational risks [6].
According to the International Tanker Owners Pollution Federation [7], the amount of oil spilled since 1970 has greatly decreased. However, the critical point mentioned by the ITOPF itself is that the statistics for small leakages (i.e., lower than 7 tons) are not precise due to the difficulty of obtaining reliable information on these events. In this context, most of the toluene and related alkylbenzenes are released to the ecosystems from gasoline and other oil products being spilled from storage tanks and pipelines [1][7]. Since these are mostly underground leaks, they remain undetected for long periods, severely affecting soil and groundwater and posing a significant ecotoxicological risk to all biological systems.
As a strategy to reduce atmospheric pollution, many countries supplement gasoline with ethanol [8]. In Brazil, commercial gasoline is a blend composed of 27% of anhydrous ethanol [9]. Toluene and other alkylbenzenes that constitute gasoline are miscible in primary alcohols such as methanol and ethanol, which are also soluble in water [10]. Thereby, the presence of ethanol allows the solubilization of high levels of alkylbenzenes [11], which, added to the aging and poor conservation of fuel storage tanks, results in leaks where ethanol carries gasoline alkylbenzenes to the soil aqueous phase. This enables alkylbenzenes to move through the soil matrix, increasing the probability of polluting underground water bodies and aquifers [4]. Such an environmental impact has been dubbed as BTEX pollution because of the predominance of benzene, toluene, ethylbenzene, and xylene isomers.
Bioremediation is an alternative technology to the usually more expensive and less environmentally sustainable physicochemical cleanup methods for treating contaminated areas. It is essentially based on the ability of microorganisms to metabolize recalcitrant and/or toxic compounds, such as aromatic hydrocarbons, by transforming them into substances with lower molecular weights that are more polar and, eventually, by completely degrading them into CO2 and H2O [12].
Filamentous fungi and yeasts have been widely studied for degrading harmful organic compounds [13][14][15][16]. Several studies point to the presence of melanized fungi (also known as black yeasts or black fungi) in environments rich in aromatic hydrocarbons, such as air biofilters for treating volatile hydrocarbons, soil contaminated with oil and gasoline spills, wood treated with creosote, and a coal-distilled fraction rich in phenolic compounds used historically as a wood preservative for railway ties and telephone poles [17][18][19][20][21]. The extremophilic nature of black fungi, in association with the recurrent isolations of their representatives in hydrocarbon-related environments, suggests their potential use in bioremediation processes.
Black fungi are a polyphyletic group that harbors several polyextremotolerant and oligotrophic species. The most evident adaptation of these ascomycetes is the production and accumulation of melanin in their cell walls. Melanin is a dark pigment that protects the cell and aids survival under a wide range of adverse conditions related to radiation and oxidative stress exposure [22][23]. Besides melanin, black fungi are able to biosynthesize other protective compounds such as mycosporines and mycosporine-like amino acids (MAAs) [22].
Gueidan et al. [24] suggested that ancestors of black fungi were originally oligotrophic organisms living on rock surfaces or subsurfaces. Currently, it is known that oligotrophic fungi can also grow in anthropogenic habitats such as glass, silicon, organic surfaces, metals [25], creosoted railway sleepers [26], and on phenolic compounds and aromatic hydrocarbons [21].
Various microbial enrichment assays based on a solid state-like protocol that used perlite as inert support incubated under a toluene atmosphere have consistently yielded melanized strains from the Exophiala and Cladophialophora genera, such as E. xenobiotica, E. bergeri, C. immunda, and C. exuberans, that are able to grow with toluene as the sole source of carbon and energy [18][20][27]. One of these strains, Cladophialophora sp. T1, later identified as C. psammophila [28], was successfully used in the biofiltration of toluene using inert packing materials [29]. As reviewed by Prenafeta-Boldú et al. [16], the assimilative toluene metabolic pathway in melanized fungi involves essentially the activity of cytochrome P-450 monooxygenase enzymes, which perform the oxidation of the methyl group as the first step in toluene degradation. Genomic studies such as that by Teixeira et al. [30] described how cytochrome P-450 genes are important for metabolizing aromatic compounds and in the process of adaptation to extreme environments. Blasi et al. [31] highlighted that cytochrome P-450 is one of the most overexpressed protein domains of C. immunda growing in the presence of toluene.
2. Isolation and Identification
A total of 200 strains were obtained from the isolation program carried out in hydrocarbon-related environments: contaminated soil, plant material, water samples, and insects. These strains were identified on morphological grounds, and identifications were confirmed through molecular means for 138 of them. A total of 17 genera and 27 species were recognized by morphological and molecular analyses, belonging to the orders Chaetothyriales (86 strains), Pleosporales (52 strains), Cladosporiales (47), Capnodiales (5 strains), Microascales (1 strain), Xylariales (1 strain), and Venturiales (1 strain). The remaining seven strains were grouped as “melanized filamentous fungi” due to the lack of molecular data and distinctive phenotypical characteristics for an accurate identification.
Figure 1 presents the correspondence between the isolated species and their environments. Species richness estimators and biodiversity indices on the isolated fungi are summarized in Table 1. The highest number of isolates (over 40 strains) was obtained from samples of the hydrocarbon-associated soil and water using the oil flotation and the pour-plate methods, respectively. However, while all identified strains from the garage soil were associated with Exophiala dermatitidis (Chaetothyriales), the highest observed richness (25 species) and biodiversity indices were observed in the water samples under the influence of an oil refinery. The strains identified from land farming and bark samples were also primarily related to the Chaetothyriales, which comprised the highest number of strains identified in the present study.
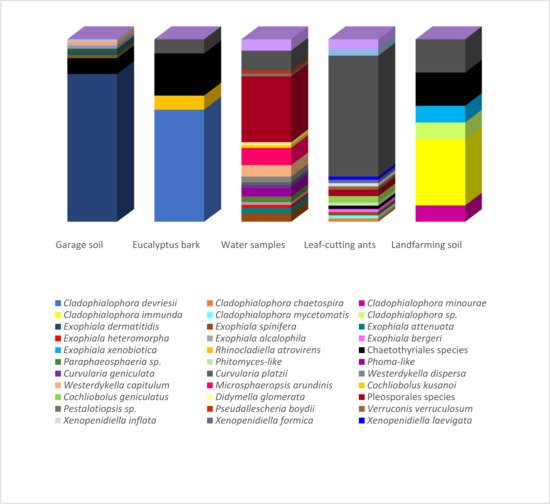
Figure 1. Relative abundance of strains obtained from the different hydrocarbon-related environments. Strains isolated from the garage soil, Eucalyptus bark, landfarming soil and leaf-cutting ants were obtained using the oil flotation technique (Satow et al., 2008). The ants were also submitted to the agar walk method. Strains recovered from the water samples were obtained by standard serial dilution according to Clesceri et al. (1998).
| Environmental Sample | Isolation Method a | No. of Strains | Observed Richness | ACE b | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|---|---|
| Eucalyptus tereticornis bark | OF | 9 | 3 | 7.07143 | 4 | 0.98643 | 0.37037 |
| Atta capiguara drone | OF, AW | 13 | 5 | 20.7037 | 11 | 1.50588 | 0.49704 |
| Atta laevigata drone | AW | 11 | 4 | 7.22469 | 4.5 | 1.49111 | 0.54545 |
| Garage soil | OF | 47 | 1 | 1 | 1 | 0 | 0 |
| Atta capiguara gyne | OF, AW | 7 | 2 | 3.11111 | 2 | 0.59167 | 0.2449 |
| Atta laevigata gyne | AW | 4 | 4 | error | 10 | 2 | 0.75 |
| Landfarming soil | OF | 7 | 4 | 5.78667 | 4.5 | 1.84237 | 0.69388 |
| Water sample | PP | 43 | 25 | 72.4482 | 47.6667 | 4.255 | 0.92699 |
Significant advances in the ecophysiology of Chaetothyrialean fungi have been achieved in the last decades [30]. This clade encompasses species that have been isolated recurrently from both clinical patients and hydrocarbon-rich environments. The connection between these two apparently distinct and highly-specific ecological traits is still a matter of discussion, and isolation campaigns such as that of the present study might provide deeper insights into the evolution of this group [32][33]. A genomic study by Moreno et al. [34] suggested an overlap between metabolic pathways used for nutrient acquisition in extreme environments and pathogenicity factors. Furthermore, it was found that black yeasts are able to undergo contractions and expansions of their genomes to increase survival according to changing environmental conditions [30][35]. Ant domatia-associated fungal species present smaller genomes than other related species in the Chaetothyriales, which might occur due to the specialized symbiosis with the insects [35]. Three genera belonging to the Herpotrichiellaceae family (Chaetothyriales) were identified among the isolates: Cladophialophora, Rhinocladiella, and Exophiala.
Cladophialophora is a monophyletic genus that comprises the bantiana and carrionii clades [30][36]. Their representatives are common human pathogens, but non-virulent environmental strains have also been reported, such as C. yegresii [37], C. hostae, C. proteae, and C. scillae [38], C. pseudocarrionii [39], C. lanosa [40], and C. psammophila [28]. In this study, the species C. devriesii, C. mycetomatis, C. chaetospira, C. minourae, and C. immunda were identified. Although commonly described as a human pathogenic black yeast, strains of C. devriesii have also been isolated from environmental samples [36], as found in this study with the eight strains from this species obtained from E. tereticornis bark. It is known that this evergreen tree is rich in natural resins that contain volatile phenolic compounds and hydrocarbons [41][42]. It is important to highlight that this is the first report of C. devriesii from Eucalyptus.
Cladophialophora mycetomatis, also found in this study, is an uncommon species originally described from the human foot after trauma with the Opuntia spine (Cactaceae) in Mexico. To date, only two publications reported its isolation: Badali et al. [36], who described the species working with strains CBS 122,637 (clinical) and CBS 454.82 (environmental), and Nascimento et al. [27], who described the isolation of this species from babassu coconut shells. In this study, one strain of C. mycetomatis was obtained from the body of an A. laevigata drone. This is the first report of this black yeast in insects and, in particular, in leaf-cutting ants of the Attini tribe. Despite the first report from a human mycetoma, only environmental strains have been obtained since then, with no disease association.
Napolitano and Juaréz [43] were the first to suggest the use of hydrocarbons from the cuticle of insects as the sole carbon source for the growth of entomopathogenic fungi on Triatoma infestans. Regarding the black fungi, several authors concluded the existence of a straight relationship between their presence and the availability of hydrocarbons as a carbon source [17][18][20]. Attili-Angelis et al. [44] described two new species of Phialophora associated with Atta spp. and addressed questions on the diversity of ant-associated Chaetothyriales and their ecological aspects. A correlation of the hydrocarbons present in the insect cuticle with the occurrence of these fungi on the ant exoskeleton was suggested, thus reaffirming the hypothesis that these molecules are a potential key for survival in this environment. In the present study, one strain of C. chaetospira, originally described as a saprophytic species [36], was isolated from ants (Figure 1).
Landfarming soil is an environment rich in petroleum hydrocarbons that receives specific management aiming at the removal of these pollutants. From these samples, one strain of C. minoure and four of C. immunda were isolated. The first is a saprophytic fungus occurring on plant debris [36] and not commonly associated with hydrocarbon-contaminated environments, unlike C. immunda, as its own name suggests an association with pollution [36]. In fact, toluene assimilation in this later fungus has been studied at the transcriptomic level [31].
A total of 57 strains were identified as Exophiala, with the representative species being E. dermatitidis (47), E. spinifera (4), E. attenuata (2), E. bergeri (1), E. heteromorpha (1), E. alcalophila (1), and E. xenobiotica (1). All the E. dermatitidis strains were isolated from the garage soil (Figure 1), indicating a high selectivity of the substrate and adaptation of the species to petroleum hydrocarbons. This species is related to human infections, but Sudhadham et al. [45] pointed out that birds, frugivorous bat feces, and fruits in tropical environments might be its environmental origin. The authors suggested that the entry into the human habitat came from the ingestion of wild fruits carrying the propagules of the species.
Exophiala alcalophila, E. heteromorpha, E. attenuata, and three of the four E. spinifera strains were obtained from the water samples (Figure 1), indicating a higher diversity of the genus in this environment. The last strain of E. spinifera and the single strain of E. bergeri were isolated from the leaf-cutting ants, while E. xenobiotica was recovered from the landfarming soil (Figure 1). The Exophiala genus is divided into several clades [46]. Exophiala heteromorpha is grouped in the dermatitidis clade, while E. xenobiotica and E. bergeri belong to the spinifera clade. These two clades contain the main lineages recognized as human pathogens, except for E. xenobiotica, originally isolated from sites contaminated with aromatic hydrocarbons [17]. No records involving E. attenuata, a representative of the mesophila clade, and E. alcalophila, which belongs to the alcalophila clade, with hydrocarbon-associated environments were found in the literature.
Two strains were identified as belonging to the Rhinocladiella genus, one of R. atrovirens and the other of R. similis, which are closely-related species. The Rhinocladiella genus is a synanamorph of Exophiala, i.e., both anamorphic species can occur at the same teleomorph. Del Palacio-Hernanz et al. [47] described the pathogenic nature of R. atrovirens; however, recent studies have been published where the species is placed as a saprotroph in the decomposition of pine wood [48][49].
The extremotolerance of melanized fungi is unquestionable. Ruibal et al. [50] isolated melanized fungi, including representatives of the Chaetothyriales order, from rock surfaces, where extreme adverse conditions such as high exposure to solar radiation and temperature, low nutrient availability, high electrolyte concentration, and low relative humidity are common. Besides the inhospitable rock surfaces, hydrocarbon-related environments such as sites contaminated with oil and its byproducts and creosote-treated wood are a great source for the isolation of these fungi [51][52], but they may also be found in unpolluted sites such as soil and vegetal debris [53]. Therefore, the general idea of their pathogenic nature must be revised, and biohazards must be assessed carefully for every new Chaetothyrialean species that displays a biotechnological potential.
The recurrent isolation of these fungi from environmental samples naturally or artificially exposed to hydrocarbons and related substrates corroborates the hypothesis of ecological dualities. This argues that strains isolated from the environment but identified as pathogenic and/or opportunistic due to a high genetic similarity might have an ecological role not related to virulence. That is, ecological niches between clinical and environmental strains related to hydrocarbons would be clearly differentiated, and the later strains would not cause infections [28].
The order Pleosporales presented the second largest number of representatives (52), isolated from all substrates (Figure 1). Pleosporales encompass a quarter of the Dothideomycetes class representatives, being the largest of its orders [54]. Pleosporalean species may occur in several habitats as epiphytes, endophytes, parasites of leaves and stems, or hyperparasites of fungi or insects, and in association with lichens or saprobes [55]. Reports of opportunistic strains are rare, which favors the exploration of their biotechnological potential, including their xenobiotic degradation capacity, which is still poorly studied in this group.
Most of the Pleosporalean strains identified in this study (44 strains out of 52) were isolated from water samples from the river under the influence of an oil refinery (Figure 1), and the eight remaining strains were obtained from the exoskeleton of leaf-cutting ants. Fungal metabolic diversity is quite significant, and the presence of alternative carbon sources that might also be toxic, such as the hydrocarbons present in these environments, could act as triggers to activate biodegradation pathways linked to detoxification and even assimilatory metabolism.
The Pleosporales anamorphs are mostly coelomycetes, but they may also be hyphomycetes. Phoma and its relatives are the most common anamorphs [55]. Boerema et al. [56] proposed a morphology-based classification of Phoma into nine sections: Phoma, Heterospora, Macrospora, Paraphoma, Peyronellaea, Phyllostictoides, Pilosa, Plenodomus, and Sclerophomella, which are precisely described in the “Phoma Identification Manual” [57]. The manual contains 223 descriptions of specific and infra-specific taxa and over 1000 synonyms in other coelomycetes genera [58]. Currently, the phylogenetic analysis of Phoma and its relatives shows the existence of several families, indicating the polyphyly of several genera and making the identification of representatives of this group more consistent [55]. In this study, 27 Pleosporalean strains were identified belonging to the genera Westerdykella (6), Microsphaeropsis (6) Cochliobolus (3), Curvularia (2), Paraphaeosphaeria (2), Epicoccum (1), Didymella (3), Pithomyces-like (1), and Phoma-like (3) (Figure 1). The 25 remaining strains were characterized as Pleosporales representatives by their morphologies. The absence of morphological characteristics (sterile colonies) and the low resolution of the DNA sequences hampered the genus/species-level identification.
Six strains were identified as Westerdykella (Sporormiaceae), including W. capitulum (4) and W. dispersa (2). The genus includes ubiquitous species occurring in manure as endophytes or in soil as saprophytes [59]. Cochliobolus kusanoi (1) and C. geniculatus (2) were isolated from water samples and the bodies of an A. capiguara drone and an A. laevigata gyne, respectively. Two Curvularia strains, namely C. platzii and C. geniculata, were also obtained from the water samples. The presence of Cochliobolus and its anamorphs Curvularia and Bipolaris had already been reported in Attini ants [60]. The three genera are members of the Pleosporaceae family and form a complex with well-known phytopathogenic species, especially of the Poaceae family (grass). Also, the Pithomyces genus is found in this family. One strain was morphologically identified as Pithomyces-like, a cosmopolitan genus found in soil and debris [61].
Six strains recovered from the water samples were identified as Microsphaeropsis arundinis, a member of the Montagnulaceae family. Alves et al. [62] described this species as an endophyte and ubiquitous. Costa et al. [63] reported its common presence in the mycobiota of Brazilian mangrove plants on the northeastern coast. Luo et al. [64] observed the production of specific sesquiterpenes by this species, referred to as “arundinols”. From the same family, two strains of Paraphaeosphaeria sp. were isolated from the same substrate.
Didymella glomerata was the representative of the Didymellaceae family, having been isolated from the water samples in this study. As with most of the Pleosporales, this species has been obtained for a long time from senescent leaves of deciduous trees [65][66]. Three other strains from the same substrate were morphologically identified as Phoma-like due to the presence of pigmented conidia, unlike what occurs with Phoma representatives. Molecular data from the ribosomal DNA did not clearly define the specific identification of the strains.
A total of 52 isolates were identified as representatives of the orders Cladosporiales (47) and Capnodiales (5). The Cladosporiales order was recently proposed, as well as the family Cladosporiaceae, in which it is included [67]. Members of Cladosporiales were previously treated as Capnodiales. Both orders belong to the Dothideomycetidae subclass and comprises epiphytes, endophytes, and saprophytes species, occurring in association with algae (forming lichens) or as parasites of fungi and animals [67][68]. In this study, all the Cladosporiales isolates belong to the Cladosporium genus. The identification to specific level was not possible since the ITS sequencing was not enough to define all species. This is a very complex and heterogeneous genus comprising hyphomycetes with a typical recognizable coronate scar type. The morphological differentiation among species is often confusing, so molecular tools are of great importance. However, even using DNA sequencing, the comparison of sequences from only one genic region may not be reliable enough for precise identification. Data from a phylogenetic analysis with some strains show that the species are allocated at the cladosporioides complex [69] within the Cladosporium genus.
Among the 47 Cladosporium strains, 37 were obtained from the exoskeleton of leaf-cutting ants. Rodrigues et al. [70][71] previously reported the presence of this genus on ants. Pagnocca et al. [72] also found the predominance of Cladosporium strains from Atta gynes, pointing out that these insects could act on its dispersion, but no interactions were shown between this genus and the symbiont fungus cultivated by the ants.
The other 10 Cladosporium strains were isolated from the water samples under the influence of an oil refinery (7), from the landfarming soil (2), and one strain from E. tereticornis bark (Figure 1). Cladosporium conidia are the most common fungal component isolated from the air [73]. It is a cosmopolitan genus with species found in all kinds of plants, debris, soil, food, paints, textiles, and any other organic matter [74]. Considering the substrates studied herein, its occurrence was quite expected. The highest number of Cladosporium species are clustered in the cladosporioides complex, and several studies report their common isolation from different environments [75][76][77], explaining the prevalence of species from this complex in the studied samples.
Representatives of the Xenopenidiella genus were obtained as members of the Capnodiales order. Only three strains were recovered from the cuticle of A. laevigata leaf-cutting ants (Figure 1). Xenopenidiella formica and X. inflata were isolated from drones, while X. laevigata was isolated from a gyne. The genus, very similar to Penidiella, was described by Quaedvlieg et al. [78] as a saprophytic on leaf litter. The presence of Xenopenidiella species associated with the ants is possibly related to their habit of foraging fresh plant matter into the nest. The molecular analysis carried in this study revealed that these three strains could represent novelties to science. Their description was made by Duarte et al. [79].
Besides these orders, one strain of Pseudallescheria boydii (from water samples—Microascales), one of Pestalotiopsis sp. (from water samples—Xylariales), and one of Verruconis verruculosum (drone of A. laevigata—Venturiales) were also isolated. The Microascales order includes saprophytic fungi commonly found in soil [80]. Pseudallescheria boydii is originally a saprophyte often isolated from agricultural soils and contaminated water; however, it has been reported from clinical samples, especially in immunosuppressed patients [81]. It is also found in hydrocarbon-related environments such as diesel pipelines and soil with oil residues, being able to degrade oil and its byproducts besides biodiesel [82][83][84][85]. Xylariales is a monophyletic order with more than 92 genera [86]. The Pestalotiopsis genus is widely spread in tropical and temperate regions and is an important plant pathogen. More than 235 species are described in this genus, and they are named according to the hosts they are associated with [87][88]. Venturiales and the Sympoventuriaceae family were described by Zhang et al. [55] in order to accommodate members of the Venturiaceae family traditionally grouped with Pleosporales. Verruconis and Ochroconis are related genera that belong to the Sympoventuriaceae family. Verruconis spp. are thermophilic, while Ochroconis spp. are mesophylic. Both genera contain clinical and environmental strains reported in the literature [89], corroborating the ecological duality already mentioned for melanized fungi.
The findings of this study illustrate the biodiversity of melanized fungi that survive in different natural and anthropogenic hydrocarbon-rich environments, and also their capacity to tolerate and even grow on toluene, supplemented as the sole carbon and energy source at relatively low and high concentrations.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9051008
References
- Mitra, S.; Roy, P. BTEX: A Serious Ground-water Contaminant. Res. J. Environ. Sci. 2011, 5, 394–398.
- Environmental Protection Agency’s (EPA). Toluene. Available online: (accessed on 30 October 2020).
- Hobara, T.; Okuda, M.; Gotoh, M.; Oki, K.; Segawa, H.; Kunitsugu, I. Estimation of the Lethal Toluene Concentration from the Accidental Death of Painting Workers. Ind. Health 2000, 38, 228–231.
- Melquiades, R.A.; Lobo, I.; Guedes, C.L.B.; Pinto, J.P. Análise de benzeno, tolueno, etilbenzeno e xilenos em solos por headspace e cromatografia gasosa/detector de ionização de chama. Semin. Ciências Exatas Tecnológicas Londrina 2006, 27, 113–120.
- Wilbur, S.M.A.; Bosh, S.B.S.U.S. Interaction Profile for: Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX); Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004.
- Occupational Safety and Health Administration (OSHA). Toluene: Occupational Exposure Limits. 2012. Available online: (accessed on 30 October 2020).
- ITOPF. Oil Tanker Spill Statistics. 2018. Available online: (accessed on 30 March 2019).
- Brito, N.N.; Zamora, P.P.; Neto, A.L.O.; De Battisti, A.; Paterniani, J.E.S.; Pelegrini, R.T. Utilização de fungos na remediação de efluentes industriais. In IV Fórum de Estudos Contábeis; Faculdades Integradas Claretianas: Rio Claro, SP, Brazil, 2002; pp. 18–22.
- Brandão, L.F.P.; Suarez, P.A.Z. Determination of the alternative butanol/gasoline and butanol/diesel fuel blends heats of combustion by a heat-loss compensated semi-microcalorimeter. J. Therm. Anal. Calorim. 2018, 132, 1953–1960.
- Silva, R.L.B.; Barra, C.M.; Monteiro, T.C.N.; Brilhante, O.M. Estudo da contaminação de poços rasos por combustíveis orgânicos e possíveis conseqüências para a saúde pública no município de Itaguaí, Rio de Janeiro, Brasil. Cad. Saúde Públ. 2002, 18, 1599–1607.
- CIMA—Conselho Interministerial do Açúcar e do Álcool. 2013. Available online: (accessed on 8 March 2018).
- Alexander, M. Biodegradation and Bioremediation, 2nd ed.; Academic Press: New York, NY, USA, 1999.
- Singh, H. Mycorremediation: Fungal Bioremediation; Wiley-Interscience, John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2006.
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Genet. 2011, 9, 177–192.
- Barrech, D.; Ali, I.; Tareen, M. A Review on Mycoremediation—The fungal bioremediation. Pure Appl. Biol. (PAB) 2018, 7, 343–348.
- Prenafeta-Boldú, F.X.; de Hoog, G.S.; Summerbell, R.C. Fungal Communities in Hydrocarbon Degradation. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; McGenity, T.J., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 1–36.
- De Hoog, G.S.; Zeng, J.S.; Harrak, M.J.; Sutton, D.A. Exophiala xenobiotica sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie Leeuwenhoek 2006, 90, 257–268.
- Prenafeta-Boldú, F.X.; Kuhn, A.; Luykx, D.M.; Anke, H.; van Groenestijn, J.W.; de Bont, J.A. Isolation and characterisation of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source. Mycol. Res. 2001, 105, 477–484.
- Prenafeta-Boldú, F.X.; Summerbell, R.; De Hoog, G.S. Fungi growing on aromatic hydrocarbons: Biotechnology’s unex-pected encounter with biohazard. FEMS Microbiol Rev. 2006, 30, 109–130.
- Zhao, J.; Zeng, J.; De Hoog, G.S.; Attili-Angelis, D.; Prenafeta-Boldú, F.X. Isolation and Identification of Black Yeasts by Enrichment on Atmospheres of Monoaromatic Hydrocarbons. Microb. Ecol. 2010, 60, 149–156.
- Isola, D.; Selbmann, L.; De Hoog, G.S.; Fenice, M.; Onofri, S.; Prenafeta-Boldú, F.X.; Zucconi, L. Isolation and Screening of Black Fungi as Degraders of Volatile Aromatic Hydrocarbons. Mycopathologia 2013, 175, 369–379.
- Gostinčar, C.; Muggia, L.; Grube, M. Polyextremotolerant black fungi: Oligotrophism, adaptive potential, and a link to lichen symbioses. Front. Microbiol. 2012, 3, 390.
- Cordero, R.J.B.; Vij, R.; Casadevall, A. Microbial melanins for radioprotection and bioremediation. Microb. Biotechnol. 2017, 10, 1186–1190.
- Gueidan, C.; Ruibal, C.; De Hoog, S.; Schneider, H. Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol. 2011, 115, 987–996.
- Gostinčar, C.; Grube, M.; Gunde-Cimerman, N. Evolution of Fungal Pathogens in Domestic Environments? Fungal Biol. 2011, 115, 1008–1018.
- Gümral, R.; Tümgör, A.; Saraçlı, M.A.; Yıldıran, Ş.T.; Ilkit, M.; de Hoog, G.S. Black yeast diversity on creosoted railway sleepers changes with ambient climatic conditions. Microb. Ecol. 2014, 68, 699–707.
- Nascimento, M.M.; Vicente, V.A.; Bittencourt, J.V.; Gelinski, J.M.L.; Prenafeta-Boldú, F.X.; Romero-Güiza, M.; Fornari, G.; Gomes, R.R.; Santos, G.D.; Ende, A.G.V.D.; et al. Diversity of opportunistic black fungi on babassu coconut shells, a rich source of esters and hydrocarbons. Fungal Biol. 2017, 121, 488–500.
- Badali, H.; Prenafeta-Boldú, F.X.; Guarro, J.; Klaassen, C.H.; Meis, J.F.; De Hoog, G.S. Cladophialophora psammophila, a novel species of Chaetothyriales with a potential use in the bioremediation of volatile aromatic hydrocarbons. Fungal Biol. 2011, 115, 1019–1029.
- Prenafeta-Boldú, F.X.; Illa, J.; Van Groenestijn, J.W.; Flotats, X. Influence of synthetic packing materials on the gas dispersion and biodegradation kinetics in fungal air biofilters. Appl. Microbiol. Biotechnol. 2008, 79, 319–327.
- Teixeira, M.M.; Moreno, L.F.; Stielow, B.; Muszewska, A.; Hainaut, M.; Gonzaga, L.; Abouelleil, A.; Patané, J.S.L.; Priest, M.; Souza, R.; et al. Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota). Stud. Mycol. 2017, 86, 1–28.
- Blasi, B.; Tafer, H.; Kustor, C.; Poyntner, C.; Lopandic, K.; Sterflinger, K. Genomic and transcriptomic analysis of the toluene degrading black yeast Cladophialophora immunda. Sci. Rep. 2017, 7, 11436.
- De Hoog, G.S.; Queiroz-Telles, F.; Haase, G.; Fernandez-Zeppenfeldt, G.; Angelis, D.A.; Van Den Ende, A.; Matos, T.; Peltroche-Llacsahuanga, H.; Pizzirani-Kleiner, A.A.; Rainer, J.; et al. Black fungi: Clinical and pathogenic approaches. Med. Mycol. 2000, 38, 243–250.
- De Hoog, G.S.; Vicente, V.A.; Gorbushina, A.A. The bright future of darkness—The rising power of black fungi: Black yeasts, microcolonial fungi, and their relatives. Mycopathologia 2013, 175, 365–368.
- Moreno, L.F.; Ahmed, A.A.O.; Brankovics, B.; Cuomo, C.A.; Menken, S.B.J.; Taj-Aldeen, S.J.; Faidah, H.; Stielow, J.B.; Teixeira, M.D.M.; Prenafeta-Boldú, F.X.; et al. Genomic Understanding of an Infectious Brain Disease from the Desert. G3 Genes Genomes Genet. 2018, 8, 909–922.
- Moreno, L.F.; Mayer, V.; Voglmayr, H.; Blatrix, R.; Stielow, J.B.; Teixeira, M.M.; Vicente, V.A.; De Hoog, S. Genomic analysis of ant domatia-associated melanized fungi (Chaetothyriales, Ascomycota). Mycol. Prog. 2019, 18, 541–552.
- Badali, H.; Gueidan, C.; Najafzadeh, M.J.; Bonifaz, A.; Gerrits Van Den Ende, A.H.G.; De Hoog, G.S. Biodiversity of Cladophialophora. Stud. Mycol. 2008, 61, 175–191.
- De Hoog, G.S.; Nishikaku, G.; Fernandez-Zeppenfeldt, C.; Padín Gonzáles, E.; Badali, H.; Richar-Yegres, N.; Gerrits Van Den Ende, H.G. Molecular nalysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Stud. Mycol. 2007, 58, 219–234.
- Crous, P.W.; Braun, U.; Schubert, K.; Groenewald, J.Z. The genus Cladosporium and similar dematiaceous hypho-mycetes. Preface Stud. Mycol. 2007, 58, 253.
- Madrid, H.; Hernandez-Restrepo, M.; Gené, J.; Cano, J.; Guarro, J.; Silva, V. New and interesting chaetothyrialean fungi from Spain. Mycol. Prog. 2016, 15, 1179–1201.
- Das, K.; Lee, S.-Y.; Jung, H.-Y. Cladophialophora lanosa sp. nov., a New Species Isolated from Soil. Mycobiology 2019, 47, 173–179.
- Nunes, T.; Pio, C. Emission of volatile organic compounds from Portuguese eucalyptus forests. Chemosphere Glob. Chang. Sci. 2001, 3, 239–248.
- Winters, A.J.; Adams, M.A.; Bleby, T.M.; Rennenberg, H.; Steigner, D.; Steinbrecher, R.; Kreuzwieser, J. Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia. Atmos. Environ. 2009, 43, 3035–3043.
- Napolitano, R.; Juaréz, N.P. Entomopathogenous fungi degrade epicuticular hydrocarbons of Triatoma infestans. Arch. Biochem. Biophys. 1997, 344, 208–214.
- Attili-Angelis, D.; Duarte AP, M.; Pagnocca, F.C.; Nagamoto, N.S.; De Vries, M.; Stielow, J.B.; de Hoog, G.S. Novel Phialophora species from leaf-cutting ants (tribe Attini). Fungal Divers. 2014, 65, 65–75.
- Sudhadham, M.; Prakitsin, S.; Sivichai, S.; Chaiyarat, R.; Dorrestein, G.; Menken, S.; de Hoog, G. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 2008, 61, 145–155.
- Li, D.M.; Li, R.Y.; De Hoog, G.; Wang, Y.X.; Wang, D.L. Exophiala asiatica, a new species from a fatal case in China. Med. Mycol. 2009, 47, 101–109.
- Del Palacio-Hernanz, A.; Moore, M.K.; Campbell, C.K.; Del Palacio-Perez-Medel, A.; Del Castillo-Cantero, R. Infection of the central nervous system by Rhinocladiella atrovirens in a patient with acquired immunodeficiency syndrome. J. Med. Vet. Mycol. 1989, 27, 127–130.
- Fukasawa, Y. Fungal succession and decomposition of Pinus densiflora snags. Ecol. Res. 2018, 33, 435–444.
- Szewczyk, W.; Kwaśna, H.; Behnke-Borowczyk, J. Fungi inhabiting knotwood of Pinus sylvestris infected by Porodaedalea pini. J. Phytopathol. 2017, 165, 500–507.
- Ruibal, C.; Gonzalo, P.; Bills, G.F. Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycol. Prog. 2005, 4, 23–38.
- Satow, M.M.; Attili-Angelis, D.; De Hoog, G.S.; Vicente, V.A. Selective factors involved in oil flotation isolation of black yeasts from the environment. Stud. Mycol. 2008, 61, 157–163.
- Vicente, V.; Attili-Angelis, D.; Pie, M.; Queiroz-Telles, F.; Cruz, L.; Najafzadeh, M.; de Hoog, G.; Zhao, J.; Pizzirani-Kleiner, A. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 2008, 61, 137–144.
- Vicente, V.A.; Angelis, D.A.D.; Queiróz-Telles Filho, F.; Pizzirani-Kleiner, A.A. Isolamento de fungos herpotriquieláceos do ambiente. Braz. J. Microbiol. 2001, 32, 47–51.
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Staplers, J.A. Dictionary of the Fungi, 10th ed.; CABI Bioscience: Wallingford, UK, 2008.
- Zhang, Y.; Crous, P.W.; Schoch, C.L.; Bahkali, A.H.; Guo, L.D.; Hyde, K.D. A molecular, morphological and ecological re-appraisal of Venturiales—A new order of Dothideomycetes. Fungal Divers. 2011, 51, 249–277.
- Boerema, H.G.; de Gruyter, J.; Noordeloos, E.M. Contributions towards a monograph of Phoma (Coelomycetes)—IV. Section Heterospora: Taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Pers. Mol. Phylogeny Evol. Fungi 1997, 16, 335–371.
- Boerema, G.H.; Gruyter, J.; Noordeloos, M.E.; Hamers, M.E.C. Phoma Identification Manual: Differentiation of Specific and Infra-Specific Taxa in C. Phoma Identification Manual: Differentiation of Specific and Infra-Specific Taxa in Culture; CABI: Wallingford, UK, 2004.
- de Gruyter, J.; Woudenberg, J.; Aveskamp, M.; Verkley, G.; Groenewald, J.; Crous, P. Redisposition of phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36.
- Ebead, G.A.; Overy, D.P.; Berrué, F.; Kerr, R.G. Westerdykella reniformis sp. nov., producing the antibiotic me-tabolites melinacidin IV and chetracin B. IMA Fungus 2012, 3, 189–201.
- Guedes, F.L.A.; Attili-Angelis, D.; Pagnocca, F.C. Selective isolation of dematiaceous fungi from the workers of Atta laevigata (Formicidae: Attini). Folia Microbiol. 2011, 57, 21–26.
- Dunn, M.; Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi. TAXON 1982, 31, 600.
- Alves, J.L.; Barreto, R.W.; Pereira, O.L.; Soares, D.J. Additions to the mycobiota of the invasive weed Miconia calvescens (Melastomataceae). Mycologia 2010, 102, 69–82.
- Costa, I.P.M.W.; Maia, L.C.; Cavalcanti, M.A. Diversity of leaf endophytic fungi in mangrove plants of Northeast Brazil. Braz. J. Microbiol. 2012, 43, 1165–1173.
- Luo, J.; Liu, X.; Li, E.; Guo, L.; Che, Y. Arundinols A–C and Arundinones A and B from the Plant Endophytic FungusMicrosphaeropsis arundinis. J. Nat. Prod. 2012, 76, 107–112.
- Luedemann, G.M. The Dictyochlamydospore of Peyronellaea glomerata (Corda) Goidanich ex Togliani Contrasted with the Dictyoporospore of Alternaria Tenuis Auct. Mycologia 1959, 51, 772–780.
- Deng, J.X.; Paul, N.C.; Li, M.J.; Seo, E.Y.; Sung, G.H.; Yu, S.H. Molecular Characterization and Morphology of Two Endophytic Peyronellaea Species from Pinus koraiensis in Korea. Mycobiology 2011, 39, 266–271.
- Abdollahzadeh, J.; Groenewald, J.Z.; Coetzee MP, A.; Wingfield, M.J.; Crous, P.W. Evolution of lifestyles in Capnodiales. Stud. Mycol. 2020, 95, 381–414.
- Crous, P.; Summerell, B.; Carnegie, A.; Wingfield, M.; Groenewald, J. Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia Mol. Phylogeny Evol. Fungi 2009, 23, 119–146.
- Bensch, K.; Groenewald, J.; Dijksterhuis, J.; Starink-Willemse, M.; Andersen, B.; Summerell, B.; Shin, H.-D.; Dugan, F.; Schroers, H.-J.; Braun, U.; et al. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol. 2010, 67, 1–94.
- Rodrigues, A.; Cable, R.N.; Mueller, U.G.; Bacci, M., Jr.; Pagnocca, F.C. Antagonistic interactions between garden yeasts and microfungal garden pathogens of leafcutting ants. Antonie Leeuwenhoek 2009, 96, 331–342.
- Rodrigues, A.; Mueller, U.G.; Ishak, H.D.; Bacci, M., Jr.; Pagnocca, F.C.; Bacci, M. Ecology of microfungal communities in gardens of fungus-growing ants (Hymenoptera: Formicidae): A year-long survey of three species of attine ants in Central Texas. FEMS Microbiol. Ecol. 2011, 78, 244–255.
- Pagnocca, F.C.; Rodrigues, A.; Nagamoto, N.S.; Bacci, M. Yeasts and filamentous fungi carried by the gynes of leaf-cutting ants. Antonie Leeuwenhoek 2008, 94, 517–526.
- Mullins, J. Microorganisms in outdoor air. In Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control, 1st ed.; Flannigan, B., Samson, R.A., Miller, J.D., Eds.; Taylor & Francis: London, UK, 2001; pp. 3–16.
- Bensch, K.; Braun, U.; Groenewald, J.; Crous, P. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401.
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but dif-ferent: The expanding realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74.
- Sandoval-Denis, M.; Gené, J.; Sutton, D.; Wiederhold, N.; Cano-Lira, J.; Guarro, J. New species of Cladosporium associated with human and animal infections. Persoonia Mol. Phylogeny Evol. Fungi 2016, 36, 281–298.
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Samson, R.A. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301.
- Quaedvlieg, W.; Binder, M.; Groenewald, J.Z.; Summerell, B.A.; Carnegie, A.J.; Burgess, T.I.; Crous, P.W. Intro-ducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia Mol. Phylogeny Evol. Fungi 2014, 33, 1.
- Duarte, A.; Attili-Angelis, D.; Baron, N.; Groenewald, J.; Crous, P.; Pagnocca, F. Riding with the ants. Persoonia Mol. Phylogeny Evol. Fungi 2017, 38, 81–99.
- Benny, G.L.; Kimbrough, J.W. A synopsis of the orders and families of Plectomycetes with keys to genera. Mycotaxon 1980, 12, 1–91.
- Bibashi, E.; De Hoog, G.S.; Kostopoulou, E.; Tsivitanidou, M.; Sevastidou, J.; Geleris, P. Invasive infection caused by Pseudallescheria boydii in an immunocompetent patient. Hippokratia 2009, 13, 184–186.
- Martin-Sanchez, P.M.; Gorbushina, A.A.; Toepel, J. Quantification of microbial load in diesel storage tanks using culture- and qPCR-based approaches. Int. Biodet. Biodegr. 2016, 126, 216–223.
- Cazarolli, J.C.; Guzatto, R.; Samios, D.; Peralba, M.D.C.R.; Cavalcanti, E.H.D.S.; Bento, F.M. Susceptibility of linseed, soybean, and olive biodiesel to growth of the deteriogenic fungus Pseudallescheria boydii. Int. Biodeterior. Biodegrad. 2014, 95, 364–372.
- Janda-Ulfig, K.; Ulfig, K.; Cano, J.; Guarro, J. A study of the growth of Pseudallescheria boydii isolates from sewage sludge and clinical sources on tributyrin, rapeseed oil, biodiesel oil and diesel oil. Ann. Agric. Environ. Med. 2008, 15, 45–49.
- April, T.M.; Abbott, S.P.; Foght, J.M.; Currah, R.S. Degradation of hydrocarbons in crude oil by the ascomycete Pseudal-lescheria boydii (Microascaceae). Can. J. Microbiol. 1998, 44, 270–278.
- Smith, G.J.; Liew, E.C.; Hyde, K.D. The Xylariales: A Monophyletic Order Containing 7 Families. Fungal Divers. 2003. Available online: (accessed on 5 May 2021).
- Maharachchikumbura, S.S.N.; Guo, L.-D.; Cai, L.; Chukeatirote, E.; Wu, W.P.; Sun, X.; Crous, P.W.; Bhat, D.J.; McKenzie, E.H.C.; Bahkali, A.H.; et al. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129.
- Maharachchikumbura, S.; Hyde, K.; Groenewald, J.; Xu, J.; Crous, P. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186.
- Samerpitak, K.; Van der Linde, E.; Choi, H.-J.; Ende, A.H.G.G.V.D.; Machouart, M.; Gueidan, C.; de Hoog, G.S. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Divers. 2014, 65, 89–126.
