Vitamin B12 is an essential water-soluble vitamin that plays a pivotal role for several physiologic functions during one’s lifespan. Specific sub-group populations, such as elderly, are at risk of vitamin B12 subclinical deficiency due to different factors. This narrative review aims to summarize facts about B12 deficiency and the burden of inadequate dietary intake in elderly population, as well as to discuss sustainable approaches to vitamin B12 deficiency in aging population.
- Vitamin B12
- cobalamin
- elderly
- micronutrient deficiency
- hidden hunger
- prevention
- sustainability
1. Introduction
Adequate intake of nutrients is essential to maintain overall health through lifespan. Growing evidence has shown that (sub)clinical nutrient inadequacies as well as deficiencies affect health and quality of life [1]. It is well known that vitamin B12 (B12) deficiency is common in specific sub-groups including the elderly [2]. Data from the National Health and Nutrition Examination Survey (NHANES) show in 6.9% and 15% prevalence of B12 deficiency in US adults respectively aged 51–70 and over 70 years [3].
Vitamin B12 is an essential water-soluble vitamin [4], also known as cobalamin, which contains ion cobalt in its structure. It is mainly found in animal products, especially meat, seafood, eggs, milk and dairy products [5]. Only microorganisms (certain archaea and bacteria [5] are able to synthetize cobalamin, thus humans obtain B12 exclusively from their diet, specifically from animal products. Plant-origin foods do not contain B12, except for algae and fortified food items (e.g., cereals, fortified substitutes of milk, flours, etc.) [6].
B12 absorption and metabolism are quite complex (as shown in Figure 1), and a number of physiological factors contribute to clinical manifestation of deficiency, frequently observed in the elderly [4]. Dietary B12 release takes place in the stomach by means of hydrochloric acid and pepsin [4]. Here, vitamin B12 is bounded to R-protein, named haptocorrin, secreted by salivary glands and stomach [4]. Once arrived in duodenum, B12 is released from its protein-complex due to pancreatic proteolytic enzymes (i.e., trypsin, chymotrypsin and elastase) [2]. Free B12 is then bound by intrinsic factor (IF), a glycoprotein secreted by parietal cells in the mucosa of the stomach [4]. B12–IF complex remains in this form until it reaches the terminal ileum where it is absorbed through endocytosis [4]. Afterward, the complex is degraded in lysosomes and vitamin B12 is eventually bound to transcobalamin (TC), forming TC-Cbl complex [2]. B12 is transported via the portal system in this complexed form [2], and it is uptaken and accumulated by body cells, where it is converted into the metabolic active forms, Methylcobalamin and Adenosylcobalamin [4].
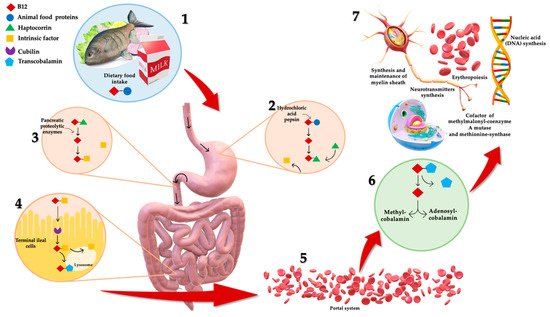
Figure 1. Complex mechanism of B12 absorption, metabolism and functions. (1) Dietary B12 is obtained through animal foods. (2) B12 release takes place in the stomach by means of hydrochloric acid and pepsin. Here, it is bounded to haptocorrin, forming a protein-complex. (3) Once arrived in duodenum, B12 is released from its protein-complex due to pancreatic proteolytic enzymes. Free B12 is then bound by intrinsic factor (IF). (4) B12–IF complex reaches terminal ileum where it is absorbed. Afterward, the complex is degraded in lysosomes and B12 is bound to transcobalamin, forming transcobalamin–B12 complex. (5) B12 is transported via the portal system in this complexed form, (6) and it is uptaken and accumulated by body cells, where it is converted to metabolic active forms: Methylcobalamin and Adenosylcobalamin. (7) B12 is crucial for several physiologic functions: erythropoiesis, synthesis and maintenance of myelin sheath, DNA and neurotransmitters synthesis, and intracytoplasmic cofactor.
Vitamin B12 is crucial for several physiologic functions, including erythropoiesis, synthesis and maintenance of myelin sheath, as well as synthesis of nucleic acid (DNA) and neurotransmitters synthesis [7][8]. It is also involved in intracytoplasmic biochemical pathways as pivotal cofactor for two enzymes: (i) the mitochondrial methylmalonyl-coenzyme A (CoA) mutase, and (ii) the cytosolic methionine-synthase [4]. The first one is involved in propionate metabolism catalyzing isomerization of methylmalonyl-CoA to succinyl-CoA; the second one is involved with other B-vitamins in the cytosolic transmethylation of homocysteine (Hcy) to methionine by 5-methyl-tetrahydrofolate [4]. During B12 deficiency, substrates of both B12-dependent reactions are accumulated, leading to increased levels of Hcy and methylmalonic acid (MMA) in plasma [2]. Excessive Hcy in blood (hyperhomocysteinemia) is associated with several chronic diseases including cardiovascular and neurodegenerative diseases, peripheral neuropathy, renal failure and hypothyroidism [9]. Hyperhomocysteinemia has also been associated to sarcopenia (defined as an age-related loss of muscle mass and function) and could mediate inhibition of the satellite cell proliferation (resident muscle precursor/stem cells with regenerative capacities properties) [10]. The latter effect is mediated by (i) enhancing the p38 MAPK signalling in different tissue types; (ii) enhancing the oxidative damage in skeletal muscles; and (iii) by inducing myostatin, an inhibitor of myogenesis, in skeletal muscles [11].
Increase in circulating MMA level is associated with an overall acidification of the body and defective fatty acid synthesis of neuronal membranes [2].
Vitamin B12 deficiency develops insidiously over the years, affecting health state. The preclinical stage of deficiency, named sub-clinical deficiency, exhibits only non-specific symptoms [12] and often remains underdiagnosed with negative health impact in vulnerable groups, especially older adults [13]. The most frequent and evident clinical manifestations of B12 deficiency are megaloblastic anemia and neurological alterations (e.g., sensory and motor disturbances, particularly in the lower extremities, ataxia) [14]. Moreover, neurological disorders can occur in the absence of hematological manifestations [2]. B12 deficiency in the elderly is also associated with cognitive decline, dementia and psychiatric disorders, covering suicidal behaviors, psychosis and mania, intense agitation [15]. Indeed, Morris M. et al. reported cognitive decline in 549 community-dwelling individuals, mean age 74.8 ± 4.6 years, having low B12 serum levels (between 187–256.8 pmol/L) [16].
Low B12 levels have also been associated with increased inflammation, oxidative stress and increased susceptibility to infections. Vitamin B12 plays an important role in gut microbioma modulation [17], which in turn impacts on the development and function of both innate and adaptive immune system [18]. Furthermore, it improves high CD4/CD8 ratio and suppresses natural killer cells [19].
There is no a consensus concerning B12 reference intervals/cut-off values; however, the recommended cut-off for its deficiency is <148 pmol/L (200 pg/mL) of plasma or serum concentration [4].
The overall long-term strategy for controlling B12 deficiency is to promote consumption of foods rich in Vitamin B12, mainly animal products [2]. Nevertheless, the elderly rarely achieve the goal due to numerous impediments including socioeconomic (cost of food) and physiological ones (i.e., swallowing and/or chewing problems), low food access (residents of food deserts), poor diets or diets lacking animal products (e.g., vegetarian and/or vegan diets), as well as ecological, religious and cultural reasons [20]. Moreover, people are driven to consume less meat by greater awareness of the negative impact on the planet besides their own health, compensated by increasing proportion of fruits, nuts and legumes [21][22][23] and consequently by increasing the risk of vitamin B12 deficiency [23].
The need to promote healthy dietary patterns [21][24][25], mainly plant-based, raises the demand to identify complementary and more sustainable sources of B12, as an alternative to animal products and to implement public health programs, to design sustainable nutritional solutions.
Based on the previous considerations, the aim of this narrative review is to describe the prevalence of B12 deficiency and the burden of its inadequate intake in the elderly, addressing the need of sustainable, public health preventive approaches to face subclinical deficiency.
2. B12 Deficiency: Dietary Intake and Bioavailability
A typical Western diet provides around 5–30 μg daily of B12, 1–5 μg of which is absorbed in the last part of the small intestine [26][27]. This amount is higher than both the Adequate Intake (AI) of B12, set at 4.0 μg for adults by the European Food Safety Authority (EFSA) (elderly included) [4] and the Recommended Dietary Allowance (RDA) set at 2.4 μg for U.S. adults by the Food and Nutrition Board [28]. Therefore, vitamin B12 deficit is rarely attributable to pure nutritional deficiency, even in the elderly [29], in western countries [30]. This hypothesis is supported by the data analysis based on nine dietary surveys conducted in Europe (Germany, Denmark, Portugal, Spain, Sweden and the UK) on 28015 adults and elderly, showing inadequate intake below 10% in elderly population (age >64 years) [31].
Economic and social factors also play a role on vitamin B12 intake first of all due to limited affordability of animal source foods (ASFs) [32]. Several studies have been conducted in low- and middle-income countries, highlighting this important association, especially in infants and children [33][34][35]. Moreover, Mark HE. et al. [36], by means of the National Food Balance Sheet 2009, which provided information on the ability of the national food supply to ensure adequate fulfillment of nutrient requirements on a population level, estimated the prevalence of micronutrient inadequacies, including vitamin A, thiamine, riboflavin, folate, vitamin B-12, zinc and calcium, in seven low-middle income countries in the South Asia regions [36].
Apart from unavailability of ASFs, inadequate intake may also be driven by religious, cultural or personal reasons (e.g., vegan diets, or less frequently, restrictive vegetarian diets [7][37]). Moreover, B12 deficiency was observed in elderly with chewing and/or swallowing impairment, restricted mobility and/or immobility, depression and social isolation [38]. All these factors are frequently associated with malnutrition [39].
Another important aspect to be considered in the elderly is food insecurity which impacts on food choices [40] and contributes to over- and under-nutrition, nutrient excesses and deficiencies [41][42]. These factors affects both low-income countries as well as high income ones [43]. Food insecurity is inversely associated with higher levels of diet quality [44], which encompasses adequacy, moderation, variety or diversity, as well as balanced nutrition and food consumption [45]. A study based on 4009 elderly adults aged 74 or more found that food insecurity was associated with a poor diet quality [46]. For instance, it can lead to a “substitution” effect [47] where nutrient-dense foods, such as lean sources of protein, are replaced with energy-dense, nutrient-poor foods, usually ultra-processed ones rich in refined carbohydrates and fats [48], giving inadequate intake of B12.
The maintenance of an optimal B12 status does not depend only on adequate dietary intake but also on B12 bioavailability in food [49]. Watanabe et al., in a study on 2861 subjects aged 71–74 years, concluded that milk and dairy products and fish were significant contributors to plasma B12, in fact vitamin B12 bioavailability from dairy products was higher than from other animal products [50]. The authors reported also a lack of association between meat and plasma B12 levels, raising doubts that cooking might contribute to significant losses (~33%) of B12, while milk and its products are largely consumed raw, safeguarding B12 bioavailability [51]. Another factor that needs to be considered is impact of heat processing of food on vitamin B12 bioavailability. Nishioka M. et al. demonstrated that B12 content of round herring meats decreased down to 59% (grilled for 7.5 min), to 47% (boiled for 5 min), to 41% (fried for 4 min), to 43% (steamed for 9 min) and to 59% (microwaved for 1 min) [52]. Evidence shows that B12 losses depend also on cooking temperature and time of cooking [52]. Heat processing of milk causes appreciable B12 losses, up to 30% and 50% when milk is boiled for 2–5 min and 30 min, respectively, 50% losses when microwaved for 5 min and 5–10% losses when pasteurized [53][54]. Those data suggested that changes in Vitamin B12 bioavailability due to time and cooking processes of food need to be considered in addition to the absolute amount of vitamin B12 content in food, when assessing dietary B12 intake.
Concerning dairy products, milk is likely to be the most important component of dairy intake impacting on serum B12 concentrations [49], in accordance with previous findings [48][51], and conflicting with others [55] that show that dairy and meat consumption were not significantly related to vitamin B12 status in a sample of 603 subjects, mean age 76.5 [55].
All in all, inadequate dietary intake is more likely to result in subclinical deficiency, revealed by biochemical markers rather than in clinical manifestation [32]. If the original B12 reserves (2–3 mg) were sufficiently large, dietary deficiency would deplete such store over several years [56].
3. Conclusions
A reduction of animal-source foods in diet is becoming more popular in western societies due to ethical, environmental, economic and health reasons, posing concerns about the beneficial or detrimental outcomes of these restrictions [37]. It is undoubtful that a dietary pattern rich in plant-based foods and poor in animal sources might benefit health and environment [22], but on the other hand it might lead to an inadequate intake of most notably vitamin B12 [2].
Such situation demands identification of sustainable sources of B12, as an alternative to exclusive meat consumption. This particularly concerns high-risk groups such as aging adults, for whom many factors apart from health concerns may contribute to the exclusion of animal source foods e.g., food pricing, age related constraints in chewing and/or swallowing, food access, as well as religious and cultural factors [20].
Given all the previous concerns, it is necessary to identify plant-source foods that naturally contain high levels of bioactive vitamin B12. Alternatively, one may consider fortification of foods [57].
Biofortification (adding vitamins and minerals to crops through plant biotechnology) is a promising approach for improving the nutritional status of a population. Moreover, the concept of in situ fortification by bacterial fermentation opens the way for innovative food products. Such strategies could be easily adopted by the food industry to develop novel vitamin B12-enhanced functional foods [58]. A long known multi-vitamin supplementation should be considered as a valid preventive treatment (even if it cannot provide the over-all long-term benefits that food-based approaches can deliver [59]). All together, these approaches would contribute to efficient measures to prevent general malnutrition in the elderly.
Global food policies involving multiple stakeholders and public–private partnership are required to help effective public health intervention to counteract expanding vitamin B12 deficiency.
This entry is adapted from the peer-reviewed paper 10.3390/nu13061913
References
- Marsman, D.; Belsky, D.W.; Gregori, D.; Johnson, M.A.; Low Dog, T.; Meydani, S.; Pigat, S.; Sadana, R.; Shao, A.; Griffiths, J.C. Healthy ageing: The natural consequences of good nutrition-a conference report. Eur. J. Nutr. 2018, 57, 15–34.
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040.
- Bird, J.K.; Murphy, R.A.; Ciappio, E.D.; McBurney, M.I. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients 2017, 9, 655.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150.
- Nohr, D.; Biesalski, H.K. Vitamin B12. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016.
- Herbert, V. Vitamin B-12: Plant sources, requirements, and assay. Am. J. Clin. Nutr. 1988, 48, 852–858.
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548.
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279.
- De Giuseppe, R.; Venturelli, G.; Guez, S.; Salera, S.; De Vita, C.; Consonni, D.; Dellanoce, C.; Bamonti, F.; Chiarelli, G.; Manzoni, F.; et al. Homocysteine metabolism in children and adolescents with epidermolysis bullosa. BMC Pediatr. 2016, 16, 173.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Veeranki, S.; Lominadze, D.; Tyagi, S.C. Hyperhomocysteinemia inhibits satellite cell regenerative capacity through p38 alpha/beta MAPK signaling. Am. J. Physiol. Heart Circ. Physiol. 2015, 15, H325–H334.
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158.
- Bailey, R.L.; West, K.P.; Black, R.E., Jr. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 22–33.
- Nawaz, A.; Khattak, N.N.; Khan, M.S.; Nangyal, H.; Sabri, S.; Shakir, M. Deficiency of vitamin B12 and its relation with neurological disorders: A critical review. J. Basic Appl. Zool. 2020, 81, 1–9.
- Mikkelsen, K.; Apostolopoulos, V. B Vitamins and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science. Subcellular Biochemistry; Harris, J., Korolchuk, V., Eds.; Springer: Singapore, 2018; Volume 90, pp. 451–470.
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464.
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health. 2020, 3, 74–92.
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441.
- Keflie, T.S.; Biesalski, H.K. Micronutrients and bioactive substances: Their potential roles in combating COVID-19. Nutrition 2021, 84, 111103.
- Porter, K.; Hoey, L.; Hughes, C.F.; Ward, M.; McNulty, H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients 2016, 8, 725.
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492.
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021. In Press.
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 Intake from Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93.
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334.
- Galimberti, A.; Cena, H.; Campone, L.; Ferri, E.; Dell’Agli, M.; Sangiovanni, E.; Belingheri, M.; Riva, M.A.; Casiraghi, M.; Labra, M. Rethinking Urban and Food Policies to Improve Citizens Safety After COVID-19 Pandemic. Front. Nutr. 2020, 7, 569542.
- Chatthanawaree, W. Biomarkers of cobalamin (vitamin B12) deficiency and its application. J. Nutr. Health Aging 2011, 15, 227–231.
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226.
- Institute of Medicine of the National Academies. Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Food and Nutrition Board; National Academies Press: Atlanta, GA, USA, 2005.
- Andrès, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Noblet-Dick, M.; Maloisel, F.; Schlienger, J.L.; Blicklé, J.F. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171, 251–259.
- Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Vitamin B12 and ageing: Current issues and interaction with folate. Ann. Clin. Biochem. 2013, 50, 315–329.
- Viñas, B.R.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.; van’t Veer, P.; Matthys, C.; Serra Majem, L. Projected prevalence of inadequate nutrient intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95.
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148 (Suppl. 4), 1995S–2027S.
- Strand, T.A.; Taneja, S.; Ueland, P.M.; Refsum, H.; Bahl, R.; Schneede, J.; Sommerfelt, H.; Bhandari, N. Cobalamin and folate status predicts mental development scores in North Indian children 12–18 mo of age. Am. J. Clin. Nutr. 2013, 97, 310–317.
- Yajnik, C.S.; Deshpande, S.S.; Lubree, H.G.; Naik, S.S.; Bhat, D.S.; Uradey, B.S.; Deshpande, J.A.; Rege, S.S.; Refsum, H.; Yudkin, J.S. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J. Assoc. Physicians India 2006, 54, 775–782.
- Ulak, M.; Chandyo, R.K.; Adhikari, R.K.; Sharma, P.R.; Sommerfelt, H.; Refsum, H.; Strand, T.A. Cobalamin and folate status in 6 to 35 months old children presenting with acute diarrhea in Bhaktapur, Nepal. PLoS ONE 2014, 9, e90079.
- Mark, H.E.; Houghton, L.A.; Gibson, R.S.; Monterrosa, E.; Kraemer, K. Estimating dietary micronutrient supply and the prevalence of inadequate intakes from national Food Balance Sheets in the South Asia regiona. Asia Pac. J. Clin. Nutr. 2016, 2, 368–376.
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.; La Ferrera, G.M.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767.
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47.
- Andrès, E.; Kaltenbach, G.; Perrin, A.E.; Kurtz, J.E.; Schlienger, J.L. Food-cobalamin malabsorption in the elderly. Am. J. Med. 2002, 113, 351–352.
- Davison, K.M.; Gondara, L.; Kaplan, B.J. Food Insecurity, Poor Diet Quality, and Suboptimal Intakes of Folate and Iron Are Independently Associated with Perceived Mental Health in Canadian Adults. Nutrients 2017, 9, 274.
- Drewnowski, A.; Specter, S.E. Poverty and obesity: The role of energy density and energy costs. Am. J. Clin. Nutr. 2004, 79, 6–16.
- Dixon, L.B.; Winkleby, M.A.; Radimer, K.L. Dietary intake and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2001, 131, 1232–1246.
- Begley, A.; Paynter, E.; Butcher, L.M.; Dhaliwal, S.S. Examining the Association between Food Literacy and Food Insecurity. Nutrients 2019, 11, 445.
- Hanson, A.D.; Beaudoin, G.A.; McCarty, D.R.; Gregory, J.F., 3rd. Does Abiotic Stress Cause Functional B Vitamin Deficiency in Plants? Plant. Physiol. 2016, 172, 2082–2097.
- Garriguet, D. Diet quality in Canada. Health Rep. 2009, 20, 41–52.
- Ford, D.W.; Hartman, T.J.; Still, C.; Wood, C.; Mitchell, D.; Hsiao, P.Y.; Bailey, R.; Smiciklas-Wright, H.; Coffman, D.L.; Jensen, G.L. Diet-related practices and BMI are associated with diet quality in older adults. Public Health Nutr. 2014, 17, 1565–1569.
- Morales, M.E.; Berkowitz, S.A. The Relationship between Food Insecurity, Dietary Patterns, and Obesity. Curr. Nutr. Rep. 2016, 5, 54–60.
- Brouwer-Brolsma, E.M.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.P.; Zwaluw, N.L.; van der Velde, N.; de Groot, L.C. Dietary Sources of Vitamin B-12 and Their Association with Vitamin B-12 Status Markers in Healthy Older Adults in the B-PROOF Study. Nutrients 2015, 7, 7781–7797.
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087.
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274.
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522.
- Nishioka, M.; Kanosue, F.; Yabuta, Y.; Watanabe, F. Loss of vitamin B(12) in fish (round herring) meats during various cooking treatments. J. Nutr. Sci. Vitaminol. 2011, 6, 432–436.
- Ball, G.F.M. Vitamin B12. In Bioavailability and Analysis of Vitamins in Foods; Chapman & Hall: London, UK, 1998; pp. 497–515.
- Watanabe, F.; Abe, K.; Fujita, T.; Goto, M.; Hiemori, M.; Nakano, Y. Effects of Microwave Heating on the Loss of Vitamin B(12) in Foods. J. Agric. Food Chem. 1998, 1, 206–210.
- Kwan, L.L.; Bermudez, O.I.; Tucker, K.L. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J. Nutr. 2002, 132, 2059–2064.
- Wong, C.W. Vitamin B12 deficiency in the elderly: Is it worth screening? Hong Kong Med. J. 2015, 21, 155–164.
- Das, J.K.; Salam, R.A.; Mahmood, S.B.; Moin, A.; Kumar, R.; Mukhtar, K.; Lassi, Z.S.; Bhutta, Z.A. Food fortification with multiple micronutrients: Impact on health outcomes in general population. Cochrane Database Syst. Rev. 2019, 12, CD011400.
- Burgess, C.M.; Smid, E.J.; van Sinderen, D. Bacterial vitamin B2, B11 and B12 overproduction: An overview. Int. J. Food Microbiol. 2009, 133, 1–7.
- Thompson, B.; Amoroso, L. Combating Micronutrient Deficiencies: Food-Based Approaches; Food and Agricultural Organisation of the United Nations: Oxfordshire, UK, 2011.
