Hepatitis D virus (HDV) is a small RNA virus with a diameter of about 36 nm, with a nucleocapsid and a 1.7Kb circular ss RNA of variable length in relation to genotype .
- HDV
- hepatitis D
- epidemiology
- pathogenesis
- therapeutics
1. Introduction
The need for the narrative review article we propose to readers stems from the extensive literature developed in recent years on different aspects of hepatitis D virus (HDV) infection. The structure and life cycle of the virus have become well known, and this has allowed the production of new agents directly acting on the HDV life cycle. Variations in HDV epidemiology have concerned countries that were already widely studied, linked in part to the intensification of migratory flows from areas at high HDV endemicity to Western countries and in part to the consistent reduction in hepatitis B virus (HBV) circulation following the worldwide application of universal HBV vaccination programs. Furthermore, new knowledge has been acquired regarding geographical areas that were poorly investigated previously.
Many reports have been published in the past on the diseases associated with HDV infection, but some clinical aspects of the more advanced phases of the illness (development of liver failure and hepatocellular carcinoma-HCC) have been more thoroughly investigated. Of great clinical relevance today is the possible diversification in the clinical expression and therapeutic response to the new antiviral agents of HDV genotypes 5–8 imported in the last two decades into Western countries from central Africa, compared to the traditional genotypes 1 and 2 [1][2][3][4][5][6].
The numerous therapeutic attempts made in the past did not provide satisfactory results. The administration of 9 million units of standard interferon-alfa (IFN-α) three times a week for 24–48 weeks provided a sustained virological response (SVR) in only 20–30% of patients treated, little in comparison to the low tolerability and the associated serious adverse reactions; it is of little wonder that this therapy became obsolete. Pegylated interferon alfa (peg-IFN-α) did not substantially improve the efficacy of the cure, although it was better tolerated and less burdened by adverse reactions. In addition, the combination of peg-IFN-α with the nucle(s)tide analogues, developed to inhibit HBV replication, did not improve the rate of SVR obtained with peg-IFN- α alone, a predictable event based on the knowledge of the HBV and HDV life cycle.
New antiviral agents directed against different replicative phases of the HDV life cycle have recently been produced and are in the initial phase of clinical experimentation, with preliminary favorable results. They too are burdened by adverse reactions, but their use in combination with other antiviral drugs seems to tone down these side effects.
2. Structure and Life Cycle of HDV
HDV is a small RNA virus with a diameter of about 36 nm, with a nucleocapsid and a 1.7Kb circular ss RNA of variable length in relation to genotype [7] (Figure 1). The HDV genome is the smallest viral genome capable of infecting mammals and looks more like viruses that infect plants than those that infect humans [8]. HDV is a defective virus that requires the HBsAg PreS-1 domain of L-HBsAg for its assembly; it lacks its own envelope, and its outer coat consists of components taken from HBV. HDV, however, does not need active HBV replication for its synthesis, because the translation of its structural proteins can also be guaranteed by the HDV RNA that is integrated alone within the hepatocytes. HDV encodes only for the antigenic protein HD-Ag, synthesized in two forms: the small HD-Ag (S-HD-Ag) and the large HD-Ag (L-HD-Ag), which are structurally identical, but L-HD-Ag has an extra 19 aa chain in the C-term [9]. HDV infects the hepatocytes through the myristoylated N-terminus of the pre-S1 domain of the L-HBsAg and its multiple transmembrane receptor sodium taurocholate co-transporting polypeptide (NTCP) [7]. After entry, HDV genome translocates into the nucleus through HD-Ag [8] and uses host RNA polymerase II, a DNA-directed RNA polymerase, to transcript HDV RNA by a rolling-circle mechanism [9] like that of plant viroids (Figure 2) [10]. First, a multimeric linear transcript is synthesized; then, using autocatalytic self-cleaving sequences, ribozymes, this linear transcript is cleaved into monomers [11]. RNA monomers are ligated by cellular RNA ligase into an antigenomic, monomeric and circular RNA used as a template for a rolling-circle replication [12]. Three different RNAs are generated: HDV genome, anti-genome and a smaller anti-genome that contains the open reading frame for HD-Ag [13][14]. S-HD-Ag has a fundamental role in replication itself, as it is required for RNA synthesis, while L-HD-Ag has a role in the packaging. Farnesylation enables the interaction between HD-Ag and HBsAg. At C-terminal for L-HD-Ag, there is a 19-amino acid polypeptide that includes C-terminal CXXX-box motif, a substrate for prenyltransferases, which adds a prenyl lipid group (farnesyl group) [15][16]. When HBsAg is completed, farnesylation anchors the protein to the endoplasmic membrane enabling the interaction through HD-Ag and HBsAg, and this allows the release of virus particles.
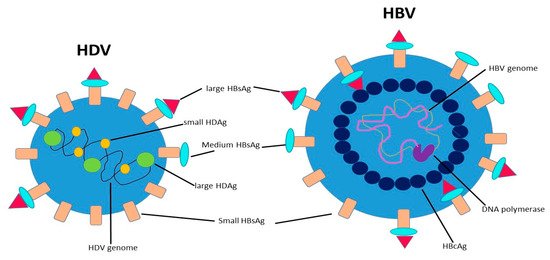
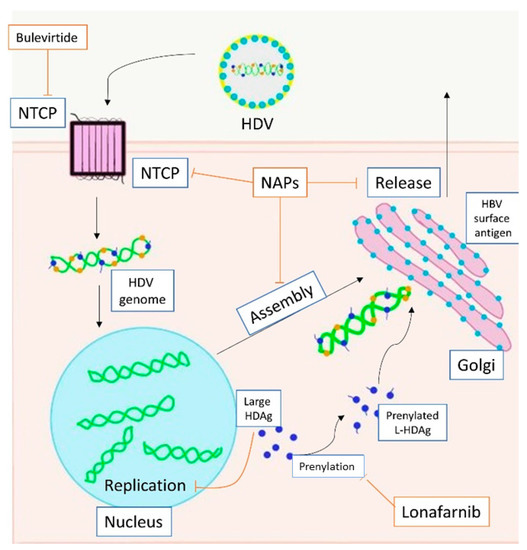
Figure 2. Action of direct anti-HDV agents on the HDV life circle. HDV enters the hepatocyte through NTCP. The replication is carried out in the nucleus. Large HDAg is farnesylated in the cytoplasm. Assembly ends in the Golgi apparatus with the bond between HBsAg and HDAg. Bulevirtide inhibits NTCP (entry), lonafarnib inhibits prenylation (farnesylation, assembly), NAPs inhibit NTCP (entry), farnesylation (assembly) and release. Footnotes: NTCP, sodium taurocholate co-transporting polypeptide; HDV, hepatitis D virus; HDAg, hepatitis D antigen; L-HDAg, large hepatits D antigen; HBV, hepatitis B virus; NAPs, nucleic acid polymers.
3. HDV Epidemiology, Unchanged Knowledge and Changes over Time
Nearly 70 million subjects are infected with HDV worldwide [17], with an anti-HDV seroprevalence among HBsAg-positive carriers varying according to the geographical area of birth, socio-economic status and exposure to risk factors [18][19]; a high prevalence of HDV chronic carriers have been observed in Central Africa, South America, Turkey, Mongolia, some Pacific island countries (Kiribati), southern Italy and the previous Soviet Union [18][20][21][22]. A systematic review and meta-analysis reported a worldwide HDV seroprevalence of 14.6% among HBV-positive patients, a percentage much higher than previous estimates of approximately 5%, with the highest seroprevalence in the intravenous drug users (IVDU), in subjects with unsafe sexual behavior and in cohabitants with HBsAg-positive family members [17].
Eight HDV genotypes have been identified, of which 7 present more than 90% similarity over the entire genomic sequence, while HDV-genotype 3 exhibits a 40% divergence with the other 7 at the nucleic acid level; in addition, 2-4 subtypes have been identified so far for each genotype [18][23][24]. HDV-genotype 1, the most represented worldwide, prevails in western Europe and North America [25]; HDV-genotype 2 was previously confined to Asia but it has recently emerged in Egypt and Iran [26][27]. HDV-genotype 3 has been found in North and South America, mostly in the Amazon basin [28], and HDV-genotype 4 has been found in China and Japan [29]. Central Africa is the main site of HDV diversification, with HDV-genotypes 1, 5, 6, 7 and 8 [29].
Several epidemiological surveys have shown an increased prevalence of HDV infection in migration-destination countries where this infection was previously uncommon [30][31][32][33][34][35]. In addition, the geographic distributions of HDV genotypes have changed over time in Western countries, mostly due to the intensification of immigration from endemic countries. In fact, HDV genotypes 5, 6 and 7 have been detected in several European countries [36][37][38]. In Australia, immigrants born in Africa have been found at higher risk of carrying HDV infection than native Australians (RR= 1.55; 95% CI 1.14–2.09) [30].]. In England, more than half the patients with HDV infection have come from continents or sub-continents where HDV infection is endemic (southern or Eastern Europe, Africa, Middle East, Asia) [32]. A similar event has occurred in Greece, where immigrants are more than half of the HDV population [34]. Changes in molecular epidemiology have also been described for HBV infection; in Italy, related to migratory flows, the “traditional” HBV genotype D has been replaced in part by other HBV genotypes in the latter decades, prevalently introduced from Africa and Eastern Europe and responsible for nearly 40% of acute hepatitis B [39][40][41][42][43]. In Italy, there was a substantial decrease in HBV endemicity consequent to changes in socio-economic conditions and to the universal HBV vaccination that started in 1991 and is still ongoing. Consequently, HDV infection among HBsAg positive subjects progressively decreased from the 24% detected in 1981 [44] and the 23.4% found in 1987 [45] to 14.4% in 1992 [46], 8.3% in 1997 [47], 8.1% in 2007 [19] and to 6.4% in 2019 [48], when, reflecting a survival effect, HDV-related chronic hepatitis affected older subjects with advanced disease. Instead, this HDV prevalence has increased in immigrants from 2001 to 2013 (from 12.2% to 26.4%), due to the increase in migratory phenomena from Africa and Eastern countries and the difficulties of vaccinating undocumented immigrants (mostly from Africa) against HBV [49][50][51][52][53][54][55][56][57].
These data suggest considering immigrants from endemic areas at high risk of being carriers of HDV infection, on a par with IVDU, men or women with multiple sexual partners and cohabitants with HBV/HDV-positive family members.
Concluding on this point, we believe that the increasing spread of HDV infection associated with migration flows is becoming a new challenge in the third millennium.
4. From HDV Infection to Liver Cirrhosis and HCC, Here Too Something New
HDV can only be transmitted in the presence of a concomitant HBV infection, by coinfection or superinfection [58]. Co-infection is the simultaneous HBV and HDV acute infection occurring in a susceptible individual. HBV/HDV coinfection is responsible for acute hepatitis resembling acute hepatitis B [59], which is frequently more severe than acute hepatitis B and provides an increased risk of acute liver failure [60][61]. As HBV infection develops before HDV infection, HBV/HDV coinfection may cause biphasic peaks in aminotransferases (AST, ALT) serum levels, even weeks apart [60][61]. As HBV is essential for HDV replication, the rate of progression to chronicity is similar to that observed in acute hepatitis B, ranging between 2% and 5% [62][63].
Chronic HBV carriers who acquire HDV infection (HDV superinfection) develop acute hepatitis Delta that progresses to chronicity in nearly three quarters of cases, frequently induces worsening of the pre-existing liver disease and the development of fulminant hepatitis in 7–15% of cases [64]. In the remaining cases HDV replication stops, allowing a less rapid, sometimes indolent course of the disease. In an Italian cohort study, 10% of HBsAg-positive patients with circulating anti-HDV antibodies cleared HBsAg during a mean follow-up period of 4 years, a percentage more than double what happens in patients with HBV mono-infection [65].
HDV chronic infection, detectable by the persistence of antibody to HDV and HDV RNA or HD-Ag in serum for at least 6 months after HDV infection, leads to chronic hepatitis Delta (HDV-CH), a disease more severe than HBV-CH, with higher aminotransferase levels and increased rates of fibrosis progression [66][67]. In addition, patients with HDV-CH are 2-fold more likely to develop and die of hepatic decompensation or HCC than those with HBV mono-infection [63][68][69]. The probability of 20-year survival after the diagnosis of HDV-CH has been estimated at 86%, the persistence of HDV replication being the only factor associated with an increased risk of mortality [63].
HBV/HDV coinfection has been identified as a main factor for the development of HCC in most studies, with a 3-fold increased risk compared to HBV infection [32][63][68][69][70][71][72][73][74]. In a few studies, however, HDV did not appear to significantly increase the risk of developing HCC [32][75]. Understanding this difference would require further investigation, but a different selection of patients by age, HDV genotype (not determined in most studies) and ethnic and environmental aspects is conceivable.
Let us focus now on the most recent data to analyze any differences compared to past. In a recent multicenter study at four hospitals in Spain, 151 (5.2%) out of 2888 HBsAg-positive subjects were anti-HDV positive, of whom 118 had a median follow-up of 8 years. Of these 118, 73% had initially detectable HDV-RNA and 30% liver cirrhosis, most often in HDV-RNA positive patients. Non-cirrhotic patients with initially detectable HDV-RNA were more prone to developing cirrhosis (31% vs. 0%, p = 0.002) and/or liver decompensation (28% vs. 3%, p = 0.019) than the HDV-RNA-negative ones [76].
A recent nationwide retrospective French study investigated 375 HDV patients with compensated cirrhosis; positive HDV-RNA at the most recent evaluation, an older age, being overweight, total serum bilirubin >17 mmol/L, and low platelet count were all identified as independent factors associated with liver decompensation. In a further analysis, the presence of HDV RNA at the most recent evaluation (HR = 2.14, p = 0.01), an older age (HR = 1.08, p < 0.001), past alcohol intake (HR = 2.39, p = 0.010), prothrombin time < 80% (HR = 4.15, p < 0.001), platelet count <100,000/mm3 (HR = 2.56, p = 0.016) and serum GGT >2-times the normal value (HR = 3.70, p = 0.002) were identified as independent factors associated with the development of HCC [77].
The long-term impact of HDV viremia on the outcome was analyzed also in a Swedish nationwide cohort of 337 HBV/HDV co-infected patients, prevalently HDV RNA positive. A significantly increased risk of liver events was seen in patients with HDV-RNA viremia at baseline (HR = 3.82, 95% CI 1.48–9.82), cirrhosis at baseline (HR = 10.26, 95% CI 5.47–19.23) and an older age (HR =1.05, 95% CI 1.03–1.08); the incidence rate per person/year was 2.81% for the HDV- RNA-positive and 0.76% for the HDV-RNA-negative. In the same cohort, a significantly higher risk of HCC development was seen in older patients (HR = 1.08 CI 95%: 1.04–1.13) (p < 0.001) and in those with cirrhosis (HR = 3.16 CI 95%: 1.22–11.13) (p = 0.02). The risk for HCC development was 0.73% per person/year for HDV-RNA-positive and 0.29% for HDV-RNA-negative patients, a difference however not reaching statistical significance [78].
The influence of the HDV genotype in the evolution of the disease is still under investigation. In a study from Taiwan, 51 patients infected with HDV genotype 1 showed a lower remission rate (15.2% vs. 40.2%; p = 0.007) and a more adverse outcomes (cirrhosis, hepatocellular carcinoma, or mortality) (52.2% vs. 25.0%; p = 0.005) than 74 patients with HDV genotype 2 [79]. Recent data from Roulot et al. indicate that European patients with HDV genotype 1 and African patients with HDV genotype 5 are at a high risk of developing cirrhosis [77]. In a small British cohort considering 21 African and 9 European patients with HBV/HDV coinfection, those born in Africa were all infected with HDV-5 and showed a better prognosis than those born in Europe, which were mostly infected with HDV-1 [80]. HDV genotype 3 has been linked to outbreaks of severe hepatitis, frequently progressing to acute liver failure and death in the Amazon Basin [28][81][82]. Genotype 4 has been found associated with a mild liver disease [83], but its variant affecting 40 Japanese patients on the Miyako Island has been found to be associated with a more rapid progression to cirrhosis than observed in Taiwan [84]. Further studies on more numerous case series will better highlight the real role of HDV genotypes in influencing the clinical course of chronic hepatitis due to HBV/HCV coinfection.
We certainly did not expect extreme variations in the clinical aspects associated with HDV infection, given the significant volume of studies in this regard produced in past years. The data reported above, however, strongly underline the persistence of HDV viremia as a strong predictor of a poor prognosis; this unfavorable influence is widely confirmed for the evolution to cirrhosis, while a moderate uncertainty persists on its effect on HCC development.
The recent increase in HDV genotypes 5–8 in Western countries is most definitely new. This has already caused and will cause even more changes in the clinical presentation and evolution of HDV-CH. It is therefore important to perform ad hoc studies to acquire rapid clinical information on HDV infection in territories where HDV genotypes 5-8 are spread.
This entry is adapted from the peer-reviewed paper 10.3390/life11020169
References
- Komas, N.P.; Ghosh, S.; Abdou-Chekaraou, M.; Pradat, P.; Al Hawajri, N.; Manirakiza, A.; Laghoe, G.L.; Békondi, C.; Brichler, S.; Ouavéné, J.-O.; et al. Hepatitis B and hepatitis D virus infections in the Central African Republic, twenty-five years after a fulminant hepatitis outbreak, indicate continuing spread in asymptomatic young adults. PLoS Negl. Trop. Dis. 2018, 12, e0006377.
- Zampino, R.; Boemio, A.; Sagnelli, C.; Alessio, L.; Adinolfi, L.E.; Sagnelli, E.; Coppola, N. Hepatitis B virus burden in developing countries. World J. Gastroenterol. 2015, 21, 11941–11953.
- Radjef, N.N.; Gordien, E.E.; Ivaniushina, V.V.; Gault, E.E.; Anaïs, P.P.; Drugan, T.T.; Trinchet, J.-C.J.-C.; Roulot, D.D.; Tamby, M.M.; Milinkovitch, M.C.; et al. Molecular Phylogenetic Analyses Indicate a Wide and Ancient Radiation of African Hepatitis Delta Virus, Suggesting a Deltavirus Genus of at Least Seven Major Clades. J. Virol. 2004, 78, 2537–2544.
- Barros, L.; Gomes-Gouvea, M.; Pinho, J.; Alvarado-Mora, M.; Dos Santos, A.; Mendes-Correa, M.; Caldas, A.; Sousa, M.; Santos, M.; Ferreira, A. Hepatitis Delta virus genotype 8 infection in Northeast Brazil: Inheritance from African slaves? Virus Res. 2011, 160, 333–339.
- Lai, A.; Sagnelli, C.; Presti, A.L.; Cella, E.; Angeletti, S.; Spoto, S.; Costantino, S.; Sagnelli, E.; Ciccozzi, M. What is changed in HBV molecular epidemiology in Italy? J. Med. Virol. 2018, 90, 786–795.
- Le Gal, F.; Gault, E.; Ripault, M.-P.; Serpaggi, J.; Trinchet, J.-C.; Gordien, E.; Deny, P. Eighth Major Clade for Hepatitis Delta Virus. Emerg. Infect. Dis. 2006, 12, 1447–1450.
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1.
- Hughes, S.A.; Wedemeyer, H.; Harrison, P.M. Hepatitis delta virus. Lancet 2011, 378, 73–85.
- Sureau, C.; Negro, F. The hepatitis delta virus: Replication and pathogenesis. J. Hepatol. 2016, 64, S102–S116.
- Branch, A.D.; Robertson, H.D. A replication cycle for viroids and other small infectious RNA’s. Science 1984, 223, 450–455.
- Macnaughton, T.B.; Wang, Y.J.; Lai, M.M. Replication of hepatitis delta virus RNA: Effect of mutations of the autocatalytic cleavage sites. J. Virol. 1993, 67.
- Reid, C.E.; Lazinski, D.W. A host-specific function is required for ligation of a wide variety of ribozyme-processed RNAs. Proc. Natl. Acad. Sci. USA 2000, 97, 424–429.
- Chang, J.; Nie, X.; Chang, H.E.; Han, Z.; Taylor, J. Transcription of Hepatitis Delta Virus RNA by RNA Polymerase II. J. Virol. 2007, 82, 1118–1127.
- Weiner, A.J.; Choo, Q.L.; Wang, K.S.; Govindarajan, S.; Redeker, A.G.; Gerin, J.L.; Houghton, M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J. Virol. 1988, 62, 594–599.
- Glenn, J.S. Prenylation of HDAg and Antiviral Drug Development. Curr. Top. Microbiol. Immunol. 2006, 307, 133–149.
- Taylor, J.M. Infection by Hepatitis Delta Virus. Viruses 2020, 12, 648.
- Chen, H.-Y.; Shen, D.-T.; Ji, D.-Z.; Han, P.-C.; Zhang, W.-M.; Ma, J.-F.; Chen, W.-S.; Goyal, H.; Pan, S.; Xu, H.-G. Prevalence and burden of hepatitis D virus infection in the global population: A systematic review and meta-analysis. Gut 2019, 68, 512–521.
- Wedemeyer, H.; Manns, M.P. Epidemiology, pathogenesis and management of hepatitis D: Update and challenges ahead. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 31–40.
- Stroffolini, T.; Almasio, P.L.; Sagnelli, E.; Mele, A.; Gaeta, G.B.; Messina, V. Evolving clinical landscape of chronic hepatitis B: A multicenter Italian study. J. Med. Virol. 2009, 81, 1999–2006.
- Jackson, K.; Tekoaua, R.; Holgate, T.; Edwards, R.; Yuen, L.; Lee, A.; Nicholson, S.; Littlejohn, M.; Locarnini, S.; Tuneti, K. Hepatitis B and D in the Pacific Islands of Kiribati. J. Clin. Virol. 2020, 129, 104527.
- Rizzetto, M.; Ponzetto, A.; Forzani, I. Hepatitis delta virus as a global health problem. Vaccine 1990, 8, S10–S14.
- Stockdale, M.A.J.; Chaponda, M.; Beloukas, A.; Phillips, R.O.; Matthews, P.C.; Papadimitropoulos, M.A.; King, S.; Bonnett, L.; Geretti, A.M. Prevalence of hepatitis D virus infection in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e992–e1003.
- Chai, N.; Chang, H.E.; Nicolas, E.; Han, Z.; Jarnik, M.; Taylor, J. Properties of Subviral Particles of Hepatitis B Virus. J. Virol. 2008, 82, 7812–7817.
- Dény, P. Hepatitis Delta Virus Genetic Variability: From Genotypes I, II, III to Eight Major Clades? Curr. Top. Microbiol. Immunol. 2006, 307, 151–171.
- Rizzetto, M.; Smedile, A. Pegylated interferon therapy of chronic hepatitis D: In need of revision. Hepatology 2015, 61, 1109–1111.
- Fouad, R.; Abdo, M.; Eldeen, H.G.; Sabry, D.; Atef, M.; Ahmed, R.; Zayed, N. Influence of delta virus infection on the virologic status in Egyptian patients with chronic hepatitis B virus genotype D. J. Med. Virol. 2015, 88, 837–842.
- Meshkat, M.; Sadeghian, H.; Esmaeelzadeh, A.; Nomani, H.; Alimardani, M.; Davoodnejad, M.; Varasteh, N.; Ahadi, M.; Sepahi, S.; Rostami, S.; et al. Distribution of Hepatitis Delta Virus Genotypes in Mashhad, Northeast Iran. Jundishapur J. Microbiol. 2015, 8.
- Casey, J.L.; Brown, T.L.; Colan, E.J.; Wignall, F.S.; Gerin, J.L. A genotype of hepatitis D virus that occurs in northern South America. Proc. Natl. Acad. Sci. USA 1993, 90, 9016–9020.
- Le Gal, F.; Dziri, S.; Gerber, A.; Alloui, C.; Ben Abdesselam, Z.; Roulot, D.; Brichler, S.; Gordien, E. Performance Characteristics of a New Consensus Commercial Kit for Hepatitis D Virus RNA Viral Load Quantification. J. Clin. Microbiol. 2016, 55, 431–441.
- Coghill, S.; McNamara, J.; Woods, M.; Hajkowicz, K. Epidemiology and clinical outcomes of hepatitis delta (D) virus infection in Queensland, Australia. Int. J. Infect. Dis. 2018, 74, 123–127.
- Shadur, B.; MacLachlan, J.; Cowie, B. Hepatitis D virus in Victoria 2000-2009. Intern. Med. J. 2013, 43, 1081–1087.
- Cross, T.J.; Rizzi, P.; Horner, M.; Jolly, A.; Hussain, M.J.; Smith, H.M.; Vergani, D.; Harrison, P.M. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J. Med. Virol. 2007, 80, 277–282.
- Kelly, V.; Kensit, J.; Barrett, A. Hepatitis D (delta) infection in south-east London. Lancet 1989, 333, 45.
- Manesis, E.K.; Vourli, G.; Dalekos, G.; Vasiliadis, T.; Manolaki, N.; Hounta, A.; Koutsounas, S.; Vafiadis, I.; Nikolopoulou, G.; Giannoulis, G.; et al. Prevalence and clinical course of hepatitis delta infection in Greece: A 13-year prospective study. J. Hepatol. 2013, 59, 949–956.
- Gheorghe, L.; Csiki, I.E.; Iacob, S.; Gheorghe, C.; Trifan, A.; Grigorescu, M.; Motoc, A.; Suceveanu, A.; Curescu, M.; Caruntu, F.; et al. Hepatitis Delta Virus Infection in Romania: Prevalence and Risk Factors. J. Gastrointest. Liver Dis. 2015, 24, 413–421.
- El Bouzidi, K.; Elamin, W.; Kranzer, K.; Irish, D.N.; Ferns, B.; Kennedy, P.; Rosenberg, W.; Dusheiko, G.; Sabin, C.A.; Smith, B.C.; et al. Hepatitis delta virus testing, epidemiology and management: A multicentre cross-sectional study of patients in London. J. Clin. Virol. 2015, 66, 33–37.
- Servant-Delmas, A.; Le Gal, F.; Gallian, P.; Gordien, E.; Laperche, S. Increasing prevalence of HDV/HBV infection over 15 years in France. J. Clin. Virol. 2014, 59, 126–128.
- Béguelin, C.A.; Moradpour, D.; Sahli, R.; Suter-Riniker, F.; Lüthi, A.; Cavassini, M.; Günthard, H.F.; Battegay, M.; Bernasconi, E.; Schmid, P.; et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J. Hepatol. 2017, 66, 297–303.
- Coppola, N.; Masiello, A.; Tonziello, G.; Pisapia, R.; Pisaturo, M.; Sagnelli, C.; Messina, V.; Iodice, V.; Sagnelli, E. Factors affecting the changes in molecular epidemiology of acute hepatitis B in a Southern Italian area. J. Viral Hepat. 2009, 17, 493–500.
- Coppola, N.; Tonziello, G.; Colombatto, P.; Pisaturo, M.; Messina, V.; Moriconi, F.; Alessio, L.; Sagnelli, E.; Cavallone, D.; Brunetto, M.R.; et al. Lamivudine-resistant HBV strain rtM204V/I in acute hepatitis B. J. Infect. 2013, 67, 322–328.
- Sagnelli, C.; Ciccozzi, M.; Pisaturo, M.; Zehender, G.; Presti, A.L.; Alessio, L.; Starace, M.; Lovero, D.; Sagnelli, E.; Coppola, N. Molecular epidemiology of hepatitis B virus genotypes circulating in acute hepatitis B patients in the Campania region. J. Med. Virol. 2014, 86, 1683–1693.
- Sagnelli, C.; Ciccozzi, M.; Coppola, N.; Minichini, C.; Presti, A.L.; Starace, M.; Alessio, L.; Macera, M.; Cella, E.; Gualdieri, L.; et al. Molecular diversity in irregular or refugee immigrant patients with HBV-genotype-E infection living in the metropolitan area of Naples. J. Med. Virol. 2017, 89, 1015–1024.
- Sagnelli, C.; Ciccozzi, M.; Alessio, L.; Cella, E.; Gualdieri, L.; Pisaturo, M.; Minichini, C.; Di Caprio, G.; Starace, M.; Onorato, L.; et al. Correction to: HBV molecular epidemiology and clinical condition of immigrants living in Italy. Infection 2019, 48, 147.
- Smedile, A.; Lavarini, C.; Farci, P.; Arico, S.; Marinucci, G.; Dentico, P.; Giuliani, G.; Cargnel, A.; Blanco, C.D.V.; Rizzetto, M. Epidemiologic patterns of infection with the hepatitis B virus-associated delta agent in Italy. Am. J. Epidemiol. 1983, 117, 223–229.
- Sagnelli, E.; Stroffolini, T.; Ascione, A.; Bonino, F.; Chiaramonte, M.; Colombo, M.; Crax, A.; Giusti, G.; Manghisi, O.G.; Pastore, G.; et al. The epidemiology of hepatitis delta infection in Italy. J. Hepatol. 1992, 15, 211–215.
- Sagnelli, E.; Stroffolini, T.; Ascione, A.; Chiaramonte, M.; Craxì, A.; Giusti, G.; Piccinino, F. Decrease in HDV endemicity in Italy. J. Hepatol. 1997, 26, 20–24.
- Gaeta, G.B.; Stroffolini, T.; Chiaramonte, M.; Ascione, T.; Stornaiuolo, G.; LoBello, S.; Sagnelli, E.; Brunetto, M.R.; Rizzetto, M. Chronic Hepatitis D: A Vanishing Disease? An Italian Multicenter Study. Hepatology 2000, 32, 824–827.
- Stroffolini, T.; Ciancio, A.; Furlan, C.; Vinci, M.; Fontana, R.; Russello, M.; Colloredo, G.; Morisco, F.; Coppola, N.; Babudieri, S.; et al. Migratory flow and hepatitis delta infection in Italy: A new challenge at the beginning of the third millennium. J. Viral Hepat. 2020, 27, 941–947.
- Coppola, N.; Alessio, L.; Onorato, L.; Sagnelli, C.; Sagnelli, E.; Pisaturo, M. HDV infection in immigrant populations. J. Med. Virol. 2019, 91, 2049–2058.
- Coppola, N.; Alessio, L.; Gualdieri, L.; Pisaturo, M.; Sagnelli, C.; Caprio, N.; Maffei, R.; Starace, M.; Angelillo, I.F.; Pasquale, G.; et al. Hepatitis B virus, hepatitis C virus and human immunodeficiency virus infection in undocumented migrants and refugees in southern Italy, January 2012 to June 2013. Eurosurveillance 2015, 20, 30009.
- Majori, S.; Baldo, V.; Tommasi, I.; Malizia, M.; Floreani, A.; Monteiro, G.; Ferrari, A.; Accordini, A.; Guzzo, P.; Baldovin, T. Hepatitis A, B, and C Infection in a Community of Sub-Saharan Immigrants Living in Verona (Italy). J. Travel Med. 2008, 15, 323–327.
- Tafuri, S.; Prato, R.; Martinelli, D.; Melpignano, L.; De Palma, M.; Quarto, M.; Germinario, C.A. Prevalence of Hepatitis B, C, HIV and syphilis markers among refugees in Bari, Italy. BMC Infect. Dis. 2010, 10, 213.
- Chironna, M.; Germinario, C.; Lopalco, P.L.; Quarto, M.; Barbuti, S. HBV, HCV and HDV infections in Albanian refugees in Southern Italy (Apulia region). Epidemiol. Infect. 2000, 125, 163–167.
- Chironna, M.; Germinario, C.; Lopalco, P.L.; Carrozzini, F.; Quarto, M. Prevalence of hepatitis virus infections in Kosovar refugees. Int. J. Infect. Dis. 2001, 5, 209–213.
- Chironna, M.; Germinario, C.A.; Lopalco, P.L.; Carrozzini, F.; Barbuti, S.; Quarto, M. Prevalence Rates of Viral Hepatitis Infections in Refugee Kurds from Iraq and Turkey. Infection 2003, 31, 70–74.
- Palumbo, E.; Scotto, G.; Faleo, G.; Cibelli, D.C.; Saracino, A.; Angarano, G. Prevalence of HBV-genotypes in immigrants affected by HBV-related chronic active hepatitis. Arq. Gastroenterol. 2007, 44, 54–57.
- Sagnelli, E. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J. Gastroenterol. 2014, 20, 7635–7643.
- Farci, P.; Niro, G.A. Clinical Features of Hepatitis D. Semin. Liver Dis. 2012, 32, 228–236.
- Smedile, A.; Verme, G.; Cargnel, A.; Dentico, P.; Opolon, P.; Vergani, D.; Farci, P.; Caredda, F.; Caporaso, N.; Trepo, C.; et al. Influence of delta infection on severity of hepatitis B. Lancet 1982, 320, 945–947.
- Pasetti, G.; Calzetti, C.; Degli Antoni, A.; Ferrari, C.; Penna, A.; Fiaccadori, F. Clinical features of hepatitis delta virus infection in a northern italian. Infection 1988, 16, 345–348.
- Raimondo, G. Multicentre study of prevalence of HBV-associated delta infection and liver disease in drug-addicts. Lancet 1982, 319, 249–251.
- Rizzetto, M.; Durazzo, M. Hepatitis delta virus (HDV) infections. J. Hepatol. 1991, 13, S116–S118.
- Romeo, R.; Del Ninno, E.; Rumi, M.; Russo, A.; SanGiovanni, A.; De Franchis, R.; Ronchi, G.; Colombo, M. A 28-Year Study of the Course of Hepatitis Δ Infection: A Risk Factor for Cirrhosis and Hepatocellular Carcinoma. Gastroenterology 2009, 136, 1629–1638.
- Farci, P.; Smedile, A.; Lavarini, C.; Piantino, P.; Crivelli, O.; Caporaso, N.; Toti, M.; Bonino, F.; Rizzetto, M. Delta hepatitis in inap-parent carriers of hepatitis B surface antigen. A disease simulating acute hepatitis B progressive to chronicity. Gastroenterology 1983, 85, 669–673.
- Niro, G.A.; Gravinese, E.; Martini, E.; Garrubba, M.; Facciorusso, D.; Conoscitore, P.; Di Giorgio, G.; Rizzetto, M.; Andriulli, A. Clearance of hepatitis B surface antigen in chronic carriers of hepatitis delta antibodies. Liver Int. 2001, 21, 254–259.
- Mathurin, P.; Thibault, V.; Kadidja, K.; Ganne-Carrié, N.; Moussalli, J.; El Younsi, M.; Di Martino, V.; Lunel, F.; Charlotte, F.; Vidaud, M.; et al. Replication status and histological features of patients with triple (B, C, D) and dual (B, C) hepatic infections. J. Viral Hepat. 2000, 7, 15–22.
- Sagnelli, E.; Felaco, F.M.; Filippini, P.; Pasquale, G.; Peinetti, P.; Buonagurio, E.; Aprea, L.; Pulella, C.; Piccinino, F.; Giusti, G. Influence of HDV infection on clinical, biochemical and histological presentation of HBsAg positive chronic hepatitis. Liver Int. 2008, 9, 229–234.
- Niro, G.A.; Smedile, A.; Ippolito, A.M.; Ciancio, A.; Fontana, R.; Olivero, A.; Valvano, M.R.; Abate, M.L.; Gioffreda, D.; Caviglia, G.P.; et al. Outcome of chronic delta hepatitis in Italy: A long-term cohort study. J. Hepatol. 2010, 53, 834–840.
- Fattovich, G.; Giustina, G.; Christensen, E.; Pantalena, M.; Zagni, I.; Realdi, G.; Schalm, S.W. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. Gut 2000, 46, 420–426.
- Verme, G.; Brunetto, M.R.; Oliveri, F.; Baldi, M.; Forzani, B.; Piantino, P.; Ponzetto, A.; Bonino, F. Role of hepatitis delta virus infection in hepatocellular carcinoma. Dig. Dis. Sci. 1991, 36, 1134–1136.
- Fattovich, G.; Boscaro, S.; Noventa, F.; Pornaro, E.; Stenico, D.; Alberti, A.; Ruol, A.; Realdi, G. Influence of Hepatitis Delta Virus Infection on Progression to Cirrhosis in Chronic Hepatitis Type B. J. Infect. Dis. 1987, 155, 931–935.
- Abbas, Z. Hepatitis D and hepatocellular carcinoma. World J. Hepatol. 2015, 7, 777–786.
- Ji, J.; Sundquist, K.; Sundquist, J. A Population-Based Study of Hepatitis D Virus as Potential Risk Factor for Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2012, 104, 790–792.
- Toukan, A.U.; Abu-El-Rub, O.A.; Abu-Laban, S.A.; Tarawneh, M.S.; Kamal, M.F.; Hadler, S.C.; Krawczynski, K.; Margolis, H.S.; Maynard, J.E. The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology 1987, 7, 1340–1345.
- Buti, M.; Homs, M.; Rodriguez-Frias, F.; Funalleras, G.; Jardí, R.; Sauleda, S.; Tabernero, D.; Schaper, M.; Esteban, R. Clinical outcome of acute and chronic hepatitis delta over time: A long-term follow-up study. J. Viral Hepat. 2011, 18, 434–442.
- Palom, A.; Rodríguez-Tajes, S.; Navascués, C.A.; García-Samaniego, J.; Riveiro-Barciela, M.; Lens, S.; Rodríguez, M.; Esteban, R.; Buti, M. Long-term clinical outcomes in patients with chronic hepatitis delta: The role of persistent viraemia. Aliment. Pharmacol. Ther. 2019, 51, 158–166.
- Roulot, D.; Brichler, S.; Layese, R.; Benabdesselam, Z.; Zoulim, F.; Thibault, V.; Scholtes, C.; Roche, B.; Castelnau, C.; Poynard, T.; et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J. Hepatol. 2020.
- Kamal, H.; Westman, G.; Falconer, K.; Duberg, A.; Weiland, O.; Haverinen, S.; Wejstål, R.; Carlsson, T.; Kampmann, C.; Larsson, S.B.; et al. Long-Term Study of Hepatitis Delta Virus Infection at Secondary Care Centers: The Impact of Viremia on Liver-Related Outcomes. Hepatology 2020, 72, 1177–1190.
- Su, C.; Huang, Y.; Huo, T.; Shih, H.H.; Sheen, I.; Chen, S.; Lee, P.; Lee, S.; Wu, J. Genotypes and Viremia of Hepatitis B and D Viruses Are Associated with Outcomes of Chronic Hepatitis D Patients. Gastroenterology 2006, 130, 1625–1635.
- Spaan, M.; Carey, I.; Wang, B.; Shang, D.; Horner, M.; Bruce, M.; Dusheiko, G.; Agarwal, K. Outcome in chronic hepatitis delta: Differences between African and non-African patients. J. Hepatol. 2017, 66, S255–S256.
- Casey, J.L.; Niro, G.A.; Engle, R.E.; Vega, A.; Gomez, H.; McCarthy, M.; Watts, D.M.; Hyams, K.C.; Gerin, J.L. Hepatitis B Virus (HBV)/Hepatitis D Virus (HDV) Coinfection in Outbreaks of Acute Hepatitis in the Peruvian Amazon Basin: The Roles of HDV Genotype III and HBV Genotype F. J. Infect. Dis. 1996, 174, 920–926.
- Wranke, A.; Borzacov, L.M.P.; Parana, R.; Lobato, C.; Hamid, S.; Ceausu, E.; Dalekos, G.N.; Rizzetto, M.; Turcanu, A.; Niro, G.A.; et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: The Hepatitis Delta International Network (HDIN). Liver Int. 2017, 38, 842–850.
- Wu, J.-C. Functional and Clinical Significance of Hepatitis D Virus Genotype II Infection. Curr. Top. Microbiol. Immunol. 2006, 307, 173–186.
- Watanabe, H.; Nagayama, K.; Enomoto, N.; Chinzei, R.; Yamashiro, T.; Izumi, N.; Yatsuhashi, H.; Nakano, T.; Robertson, B.H.; Nakasone, H.; et al. Chronic hepatitis delta virus infection with genotype IIb variant is correlated with progressive liver disease. J. Gen. Virol. 2003, 84, 3275–3289.
