AIDS first emerged decades ago; however, its cure, i.e., eliminating all virus sources, is still unachievable.
- HIV-1
- latently HIV-1-infected cell
- latency-reversing agent
- BET protein
- BRD2
- BRD4
- LRA
- BETi
- epigenetics
- immune response
1. Overview
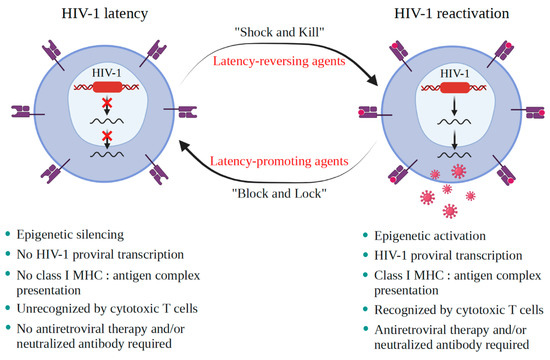
AIDS first emerged decades ago; however, its cure, i.e., eliminating all virus sources, is still unachievable. A critical burden of AIDS therapy is the evasive nature of HIV-1 in face of host immune responses, the so-called “latency.” Recently, a promising approach, the “Shock and Kill” strategy, was proposed to eliminate latently HIV-1-infected cell reservoirs. The “Shock and Kill” concept involves two crucial steps: HIV-1 reactivation from its latency stage using a latency-reversing agent (LRA) followed by host immune responses to destroy HIV-1-infected cells in combination with reinforced antiretroviral therapy to kill the progeny virus. Hence, a key challenge is to search for optimal LRAs. Looking at epigenetics of HIV-1 infection, researchers proved that some bromodomains and extra-terminal motif protein inhibitors (BETis) are able to reactivate HIV-1 from latency. However, to date, only a few BETis have shown HIV-1-reactivating functions, and none of them have yet been approved for clinical trial. In this review, we aim to demonstrate the epigenetic roles of BETis in HIV-1 infection and HIV-1-related immune responses. Possible future applications of BETis and their HIV-1-reactivating properties are summarized and discussed.
2. Background
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/v13061026
References
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161.
- Massanella, M.; Fromentin, R.; Chomont, N. Residual inflammation and viral reservoirs: Alliance against an HIV cure. Curr. Opin. HIV AIDS 2016, 11, 234.
- Ait-Ammar, A.; Kula, A.; Darcis, G.; Verdikt, R.; de Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Rohr, O.; van Lint, C. Current status of latency reversing agents facing the heterogeneity of HIV-1 cellular and tissue reservoirs. Front. Microbiol. 2020, 10, 3060.
- Marcello, A. Latency: The hidden HIV-1 challenge. Retrovirology 2006, 3, 7.
- García, M.; Buzón, M.J.; Benito, J.M.; Rallón, N. Peering into the HIV reservoir. Rev. Med. Virol. 2018, 28, e1981.
- Sung, J.M.; Margolis, D.M. HIV persistence on antiretroviral therapy and barriers to a cure. Adv. Exp. Med. Biol. 2018, 1075, 165–185.
- Eisele, E.; Siliciano, R.F. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012, 37, 377–388.
- Le Douce, V.; Ait-Amar, A.; Forouzanfar, F.; Fahmi, F.; Quiel, J.; El Mekdad, H.; Daouad, F.; Marban, C.; Rohr, O.; Schwartz, C. Improving combination antiretroviral therapy by targeting HIV-1 gene transcription. Expert Opin. Ther. Targets 2016, 20, 1311–1324.
- Le Douce, V.; Cherrier, T.; Riclet, R.; Rohr, O.; Schwartz, C. The many lives of CTIP2: From AIDS to cancer and cardiac hypertrophy. J. Cell. Physiol. 2014, 229, 533–537.
- Le Douce, V.; Forouzanfar, F.; Eilebrecht, S.; van Driessche, B.; Ait-Ammar, A.; Verdikt, R.; Kurashige, Y.; Marban, C.; Gautier, V.; Candolfi, E.; et al. HIC1 controls cellular- and HIV-1-gene transcription via interactions with CTIP2 and HMGA1. Sci. Rep. 2016, 6, 1–14.
- Marban, C.; Forouzanfar, F.; Ait-Ammar, A.; Fahmi, F.; El Mekdad, H.; Daouad, F.; Rohr, O.; Schwartz, C. Targeting the brain reservoirs: Toward an HIV cure. Front. Immunol. 2016, 7, 397.
- Marban, C.; Suzanne, S.; Dequiedt, F.; de Walque, S.; Redel, L.; van Lint, C.; Aunis, D.; Rohr, O. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007, 26, 412–423.
- Cherrier, T.; Suzanne, S.; Redel, L.; Calao, M.; Marban, C.; Samah, B.; Mukerjee, R.; Schwartz, C.; Gras, G.; Sawaya, B.E.; et al. p21WAF1 gene promoter is epigenetically silenced by CTIP2 and SUV39H1. Oncogene 2009, 28, 3380–3389.
- Le Douce, V.; Colin, L.; Redel, L.; Cherrier, T.; Herbein, G.; Aunis, D.; Rohr, O.; van Lint, C.; Schwartz, C. LSD1 cooperates with CTIP2 to promote HIV-1 transcriptional silencing. Nucleic Acids Res. 2012, 40, 1904–1915.
- Cherrier, T.; Le Douce, V.; Eilebrecht, S.; Riclet, R.; Marban, C.; Dequiedt, F.; Goumon, Y.; Paillart, J.C.; Mericskay, M.; Parlakian, A.; et al. CTIP2 is a negative regulator of P-TEFb. Proc. Natl. Acad. Sci. USA 2013, 110, 12655–12660.
- Eilebrecht, S.; Le Douce, V.; Riclet, R.; Targat, B.; Hallay, H.; van Driessche, B.; Schwartz, C.; Robette, G.; van Lint, C.; Rohr, O.; et al. HMGA1 recruits CTIP2-repressed P-TEFb to the HIV-1 and cellular target promoters. Nucleic Acids Res. 2014, 42, 4962–4971.
- Darcis, G.; van Driessche, B.; van Lint, C. HIV latency: Should we shock or lock? Trends Immunol. 2017, 38, 217–228.
- Elsheikh, M.M.; Tang, Y.; Li, D.; Jiang, G. Deep latency: A new insight into a functional HIV cure. EBioMedicine 2019, 45, 624–629.
- Mousseau, G.; Aneja, R.; Clementz, M.A.; Mediouni, S.; Lima, N.S.; Haregot, A.; Kessing, C.F.; Jablonski, J.A.; Thenin-Houssier, S.; Nagarsheth, N.; et al. Resistance to the Tat inhibitor didehydro-cortistatin A is mediated by heightened basal HIV-1 transcription. mBio 2019, 10, e01750-18.
- Planas, D.; Pagliuzza, A.; Ponte, R.; Fert, A.; Marchand, L.R.; Massanella, M.; Gosselin, A.; Mehraj, V.; Dupuy, F.P.; Isnard, S.; et al. LILAC pilot study: Effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy. EBioMedicine 2021, 65, 103270.
- Rohr, O. Flower power: Locking HIV in the gut with French lilac. EBioMedicine 2021, 66, 103299.
- Schwartz, C.; Bouchat, S.; Marban, C.; Gautier, V.; van Lint, C.; Rohr, O.; Le Douce, V. On the way to find a cure: Purging latent HIV-1 reservoirs. Biochem. Pharmacol. 2017, 146, 10–22.
- Abner, E.; Jordan, A. HIV “shock and kill” therapy: In need of revision. Antiviral Res. 2019, 166, 19–34.
