The identification of thrombospondin-1 as an angiogenesis inhibitor in 1990 prompted interest in its role in cancer biology and potential as a therapeutic target. Decreased thrombospondin-1 mRNA and protein expression are associated with progression in several cancers, while expression by nonmalignant cells in the tumor microenvironment and circulating levels in cancer patients can be elevated. THBS1 is not a tumor suppressor gene, but the regulation of its expression in malignant cells by oncogenes and tumor suppressor genes mediates some of their effects on carcinogenesis, tumor progression, and metastasis. In addition to regulating angiogenesis and perfusion of the tumor vasculature, thrombospondin-1 limits antitumor immunity by CD47-dependent regulation of innate and adaptive immune cells. Conversely, thrombospondin-1 is a component of particles released by immune cells that mediate tumor cell killing. Thrombospondin-1 differentially regulates the sensitivity of malignant and nonmalignant cells to genotoxic stress caused by radiotherapy and chemotherapy.
- angiogenesis
- cytotoxic T cells
- natural killer cells
- autophagy
- nitric oxide
- carcinogenesis
1. Introduction
2. TSP1 Regulation of Angiogenesis and Tumor Perfusion
2.1. Inhibition and Stimulation of Angiogenesis
2.2. Vascular Perfusion of Tumors and the Steal Effect
2.3. Endothelial Cell Apoptosis
3 TSP1 and Antitumor Immunity
3.1. Regulation of T Cell Immunity
3.2. TSP1 Regulation of Innate Immune Cells
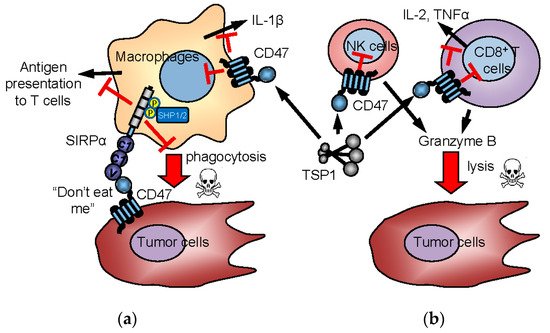
3.3. CD47 and TSP1 Signaling in Macrophages
3.4. Intrinsic Functions of CD47 in NK Cells
3.5. TSP1 in Supramolecular Attack Particles
4. TSP1 and Carcinogenesis
Several studies support roles for TSP1 in carcinogenesis. Targeted overexpression of TSP1 in the epidermis delayed and reduced premalignant epithelial hyperplasias induced by chemical carcinogens [125]. This protective role of TSP1 over-expression was extended to UVB-induced skin carcinogenesis [126] and spontaneous mammary adenocarcinomas in TgN-neu mice [127]. Conversely, loss of Thbs1 increased mammary adenocarcinomas in these mice [127] and increased osteosarcoma incidence but not the incidence of some other malignancies in mice lacking p53 [19]. In a murine model of prostate cancer metastasis to bone, increased TSP1 was observed in platelets, and implantation of tumors in Thbs1 null mice resulted in increased tumor size [128]. On the other hand, the absence of TSP1 reduced bone marrow-derived cell mobilization and enhanced osteoclast formation, resulting in decreased tumor-induced bone formation, suggesting a role of TSP1 in the pre-metastatic niche formation.
TSP1 limited angiogenesis and inflammatory responses that contribute to colorectal carcinogenesis in ApcMin/+ mice [129] and colorectal carcinogenesis induced by chronic inflammation [130]. The ability of TSP1 to regulate the responses of cells and tissues to stress prompted us to examine whether loss of TSP1 also has systemic effects on metabolism that modulate carcinogenesis [131]. ApcMin/+:Thbs1−/− mice exhibited decreased survival and higher tumor multiplicities in the small and large intestine relative to ApcMin/+ mice when fed a low-fat Western diet. However, the protective effect of endogenous TSP1 was lost when the mice were fed a high-fat Western diet. Biochemical profiles of liver tissue identified systemic metabolic changes associated with the effects of TSP1 and dietary lipid intake on tumorigenesis. A high-fat Western diet differentially regulated amino acid, energy, and lipid metabolism in ApcMin/+:Thbs1−/− mice relative to ApcMin/+ mice. Changes in ketone body and tricarboxylic acid cycle intermediates identified functional interactions between Apc and TSP1 signaling that control mitochondrial function. These data suggest that the protective role of TSP1 to limit adenoma formation in ApcMin/+ mice results in part from improved mitochondrial function and eicosanoid signaling [132].
Conclusions
The apparent contradictions that have emerged from efforts to define the role of TSP1 in the tumor microenvironment are understandable considering its interactions with multiple signaling receptors and with angiogenic and immune-modulatory factors in the extracellular matrix. To date, efforts to develop therapeutics have targeted the TSP1 receptors CD36 and CD47. Although TSP1 mimetics targeting CD36 showed antitumor efficacy in mice and dogs, these have not proven effective in human clinical trials [133][134][135]. CD47-targeted antibodies have shown more promising results in human clinical trials [105][106][107][108][109], but it remains unclear whether modulating TSP1 signaling plays any role in their efficacy. However, preclinical studies using the CD47 antibody B6H12 indicate that some CD47 antibodies can block both TSP1 and SIRPα interactions with CD47 [105]. Next generation CD47-targeted agents that selectively disrupt the TSP1-CD47 versus the CD47-SIRPα interaction are needed to further differentiate CD47 receptor biology and remain an focus of our ongoing work [136].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094570
References
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659.
- Zabrenetzky, V.; Harris, C.C.; Steeg, P.S.; Roberts, D.D. Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int. J. Cancer 1994, 59, 191–195.
- Weinstat-Saslow, D.L.; Zabrenetzky, V.S.; VanHoutte, K.; Frazier, W.A.; Roberts, D.D.; Steeg, P.S. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994, 54, 6504–6511.
- Kragh, M.; Quistorff, B.; Tenan, M.; Van Meir, E.G.; Kristjansen, P.E. Overexpression of thrombospondin-1 reduces growth and vascular index but not perfusion in glioblastoma. Cancer Res. 2002, 62, 1191–1195.
- Jin, R.J.; Kwak, C.; Lee, S.G.; Lee, C.H.; Soo, C.G.; Park, M.S.; Lee, E.; Lee, S.E. The application of an anti-angiogenic gene (thrombospondin-1) in the treatment of human prostate cancer xenografts. Cancer Gene 2000, 7, 1537–1542.
- Streit, M.; Velasco, P.; Brown, L.F.; Skobe, M.; Richard, L.; Riccardi, L.; Lawler, J.; Detmar, M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am. J. Pathol. 1999, 155, 441–452.
- Wu, M.P.; Young, M.J.; Tzeng, C.C.; Tzeng, C.R.; Huang, K.F.; Wu, L.W.; Chou, C.Y. A novel role of thrombospondin-1 in cervical carcinogenesis: Inhibit stroma reaction by inhibiting activated fibroblasts from invading cancer. Carcinogenesis 2008, 29, 1115–1123.
- Janz, A.; Sevignani, C.; Kenyon, K.; Ngo, C.V.; Thomas-Tikhonenko, A. Activation of the myc oncoprotein leads to increased turnover of thrombospondin-1 mRNA. Nucleic Acids Res. 2000, 28, 2268–2275.
- Giuriato, S.; Ryeom, S.; Fan, A.C.; Bachireddy, P.; Lynch, R.C.; Rioth, M.J.; van Riggelen, J.; Kopelman, A.M.; Passegue, E.; Tang, F.; et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc. Natl. Acad. Sci. USA 2006, 103, 16266–16271.
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of nitric oxide signalling by thrombospondin 1: Implications for anti-angiogenic therapies. Nat. Rev. Cancer 2009, 9, 182–194.
- Lawler, J.; Detmar, M. Tumor progression: The effects of thrombospondin-1 and -2. Int. J. Biochem. Cell Biol. 2004, 36, 1038–1045.
- Doci, C.L.; Zhou, G.; Lingen, M.W. The novel tumor suppressor NOL7 post-transcriptionally regulates thrombospondin-1 expression. Oncogene 2013, 32, 4377–4386.
- Stenina-Adognravi, O. Invoking the power of thrombospondins: Regulation of thrombospondins expression. Matrix Biol. 2014, 37, 69–82.
- Watnick, R.S.; Rodriguez, R.K.; Wang, S.; Blois, A.L.; Rangarajan, A.; Ince, T.; Weinberg, R.A. Thrombospondin-1 repression is mediated via distinct mechanisms in fibroblasts and epithelial cells. Oncogene 2015, 34, 2949–2950.
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gutgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231.
- Isenberg, J.S.; Roberts, D.D. THBS1 (thrombospondin-1). Atlas Genet. Cytogenet. Oncol. Haematol. 2020, 24, 291.
- Sweetwyne, M.T.; Murphy-Ullrich, J.E. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012, 31, 178–186.
- Riss, J.; Khanna, C.; Koo, S.; Chandramouli, G.V.; Yang, H.H.; Hu, Y.; Kleiner, D.E.; Rosenwald, A.; Schaefer, C.F.; Ben-Sasson, S.A.; et al. Cancers as wounds that do not heal: Differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res. 2006, 66, 7216–7224.
- Lawler, J.; Miao, W.M.; Duquette, M.; Bouck, N.; Bronson, R.T.; Hynes, R.O. Thrombospondin-1 gene expression affects survival and tumor spectrum of p53-deficient mice. Am. J. Pathol. 2001, 159, 1949–1956.
- Baek, K.H.; Bhang, D.; Zaslavsky, A.; Wang, L.C.; Vachani, A.; Kim, C.F.; Albelda, S.M.; Evan, G.I.; Ryeom, S. Thrombospondin-1 mediates oncogenic Ras-induced senescence in premalignant lung tumors. J. Clin. Investig. 2013, 123, 4375–4389.
- Taraboletti, G.; Roberts, D.; Liotta, L.A.; Giavazzi, R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: A potential angiogenesis regulatory factor. J. Cell Biol. 1990, 111, 765–772.
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J. Biol. Chem. 2010, 285, 38923–38932.
- Bagavandoss, P.; Wilks, J.W. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem. Biophys. Res. Commun. 1990, 170, 867–872.
- Good, D.J.; Polverini, P.J.; Rastinejad, F.; Le, B.M.; Lemons, R.S.; Frazier, W.A.; Bouck, N.P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA 1990, 87, 6624–6628.
- Guo, N.; Krutzsch, H.C.; Inman, J.K.; Roberts, D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997, 57, 1735–1742.
- Iruela-Arispe, M.L.; Lombardo, M.; Krutzsch, H.C.; Lawler, J.; Roberts, D.D. Inhibition of angiogenesis by thrombspondin-1 is mediated by two independent regions within the type 1 repeats. Circulation 1999, 100, 1423–1431.
- Isenberg, J.S.; Ridnour, L.A.; Perruccio, E.M.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 13141–13146.
- Kazerounian, S.; Yee, K.O.; Lawler, J. Thrombospondins in cancer. Cell. Mol. Life Sci. 2008, 65, 700–712.
- Dawson, D.W.; Pearce, S.F.; Zhong, R.; Silverstein, R.L.; Frazier, W.A.; Bouck, N.P. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 1997, 138, 707–717.
- Haviv, F.; Bradley, M.F.; Kalvin, D.M.; Schneider, A.J.; Davidson, D.J.; Majest, S.M.; McKay, L.M.; Haskell, C.J.; Bell, R.L.; Nguyen, B.; et al. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: Design, synthesis, and optimization of pharmacokinetics and biological activities. J. Med. Chem. 2005, 48, 2838–2846.
- DiPietro, L.A.; Nissen, N.N.; Gamelli, R.L.; Koch, A.E.; Pyle, J.M.; Polverini, P.J. Thrombospondin 1 synthesis and function in wound repair. Am. J. Pathol. 1996, 148, 1851–1860.
- Agah, A.; Kyriakides, T.R.; Lawler, J.; Bornstein, P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am. J. Pathol. 2002, 161, 831–839.
- Kyriakides, T.R.; Leach, K.J.; Hoffman, A.S.; Ratner, B.D.; Bornstein, P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl. Acad. Sci. USA 1999, 96, 4449–4454.
- Bornstein, P.; Agah, A.; Kyriakides, T.R. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int. J. Biochem. Cell Biol. 2004, 36, 1115–1125.
- Krady, M.M.; Zeng, J.; Yu, J.; MacLauchlan, S.; Skokos, E.A.; Tian, W.; Bornstein, P.; Sessa, W.C.; Kyriakides, T.R. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 2008, 173, 879–891.
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 212–230.
- Isenberg, J.S.; Hyodo, F.; Matsumoto, K.; Romeo, M.J.; Abu-Asab, M.; Tsokos, M.; Kuppusamy, P.; Wink, D.A.; Krishna, M.C.; Roberts, D.D. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 2007, 109, 1945–1952.
- Isenberg, J.S.; Maxhimer, J.B.; Powers, P.; Tsokos, M.; Frazier, W.A.; Roberts, D.D. Treatment of liver ischemia/reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 2008, 144, 752–761.
- Isenberg, J.S.; Pappan, L.K.; Romeo, M.J.; Abu-Asab, M.; Tsokos, M.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann. Surg. 2008, 247, 180–190.
- Isenberg, J.S.; Romeo, M.J.; Abu-Asab, M.; Tsokos, M.; Oldenborg, A.; Pappan, L.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Increasing survival of ischemic tissue by targeting CD47. Circ. Res. 2007, 100, 712–720.
- Isenberg, J.S.; Romeo, M.J.; Maxhimer, J.B.; Smedley, J.; Frazier, W.A.; Roberts, D.D. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: Implications for human disease. Ann. Surg. 2008, 247, 860–868.
- Isenberg, J.S.; Hyodo, F.; Pappan, L.K.; Abu-Asab, M.; Tsokos, M.; Krishna, M.C.; Frazier, W.A.; Roberts, D.D. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2582–2588.
- Maxhimer, J.B.; Shih, H.B.; Isenberg, J.S.; Miller, T.W.; Roberts, D.D. Thrombospondin-1-CD47 blockade following ischemia reperfusion injury is tissue protective. Plast. Reconstr. Surg. 2009, 124, 1880–1889.
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 2006, 281, 26069–26080.
- Isenberg, J.S.; Qin, Y.; Maxhimer, J.B.; Sipes, J.M.; Despres, D.; Schnermann, J.; Frazier, W.A.; Roberts, D.D. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009, 28, 110–119.
- Isenberg, J.S.; Romeo, M.J.; Yu, C.; Yu, C.K.; Nghiem, K.; Monsale, J.; Rick, M.E.; Wink, D.A.; Frazier, W.A.; Roberts, D.D. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 2008, 111, 613–623.
- Isenberg, J.S.; Hyodo, F.; Ridnour, L.A.; Shannon, C.S.; Wink, D.A.; Krishna, M.C.; Roberts, D.D. Thrombospondin-1 and vasoactive agents indirectly alter tumor blood flow. Neoplasia 2008, 10, 886–896.
- Ehrenfeld, W.K.; Harris, J.D.; Wylie, E.J. Vascular “steal” phenomenon. An experimental study. Am. J. Surg. 1968, 116, 192–197.
- Jimenez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000, 6, 41–48.
- Freyberg, M.A.; Kaiser, D.; Graf, R.; Buttenbender, J.; Friedl, P. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem. Biophys. Res. Commun. 2001, 286, 141–149.
- Rege, T.A.; Stewart, J., Jr.; Dranka, B.; Benveniste, E.N.; Silverstein, R.L.; Gladson, C.L. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J. Cell. Physiol. 2009, 218, 94–103.
- Hamano, Y.; Sugimoto, H.; Soubasakos, M.A.; Kieran, M.; Olsen, B.R.; Lawler, J.; Sudhakar, A.; Kalluri, R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004, 64, 1570–1574.
- Li, Z.; He, L.; Wilson, K.E.; Roberts, D.D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J. Immunol. 2001, 166, 2427–2436.
- Li, Z.; Calzada, M.J.; Sipes, J.M.; Cashel, J.A.; Krutzsch, H.C.; Annis, D.; Mosher, D.F.; Roberts, D.D. Interactions of thrombospondins with α4β1 integrin and CD47 differentially modulate T cell behavior. J. Cell Biol. 2002, 157, 509–519.
- Kaur, S.; Kuznetsova, S.; Pendrak, M.; Sipes, J.; Romeo, M.; Li, Z.; Zhang, L.; Roberts, D. Heparan Sulfate Modification of the Transmembrane Receptor CD47 Is Necessary for Inhibition of T Cell Receptor Signaling by Thrombospondin-1. J. Biol. Chem. 2011, 286, 14991–15002.
- Miller, T.W.; Kaur, S.; Ivins-O’Keefe, K.; Roberts, D.D. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013, 32, 316–324.
- Lamy, L.; Foussat, A.; Brown, E.J.; Bornstein, P.; Ticchioni, M.; Bernard, A. Interactions between CD47 and thrombospondin reduce inflammation. J. Immunol. 2007, 178, 5930–5939.
- Manna, P.P.; Frazier, W.A. The mechanism of CD47-dependent killing of T cells: Heterotrimeric Gi-dependent inhibition of protein kinase A. J. Immunol. 2003, 170, 3544–3553.
- Grimbert, P.; Bouguermouh, S.; Baba, N.; Nakajima, T.; Allakhverdi, Z.; Braun, D.; Saito, H.; Rubio, M.; Delespesse, G.; Sarfati, M. Thrombospondin/CD47 interaction: A pathway to generate regulatory T cells from human CD4+ CD25− T cells in response to inflammation. J. Immunol. 2006, 177, 3534–3541.
- Kvansakul, M.; Adams, J.C.; Hohenester, E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. EMBO J. 2004, 23, 1223–1233.
- Sipes, J.M.; Krutzsch, H.C.; Lawler, J.; Roberts, D.D. Cooperation between thrombospondin-1 type 1 repeat peptides and integrin αvβ3 ligands to promote melanoma cell spreading and focal adhesion formation. J. Biol. Chem. 1999, 274, 22755–22762.
- Barazi, H.O.; Li, Z.; Cashel, J.A.; Krutzsch, H.C.; Annis, D.S.; Mosher, D.F.; Roberts, D.D. Regulation of integrin function by CD47 ligands. Differential effects on αvβ3 and α4β1 integrin-mediated adhesion. J. Biol. Chem. 2002, 277, 42859–42866.
- Li, S.S.; Liu, Z.; Uzunel, M.; Sundqvist, K.G. Endogenous thrombospondin-1 is a cell surface ligand for regulation of integrin dependent T lymphocyte adhesion. Blood 2006, 108, 3112–3120.
- Kaur, S.; Singh, S.P.; Elkahloun, A.G.; Wu, W.; Abu-Asab, M.S.; Roberts, D.D. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014, 37, 49–59.
- Kaur, S.; Chang, T.; Singh, S.P.; Lim, L.; Mannan, P.; Garfield, S.H.; Pendrak, M.L.; Soto-Pantoja, D.R.; Rosenberg, A.Z.; Jin, S.; et al. CD47 signaling regulates the immunosuppressive activity of VEGF in T cells. J. Immunol. 2014, 193, 3914–3924.
- Miller, T.W.; Wang, E.A.; Gould, S.; Stein, E.V.; Kaur, S.; Lim, L.; Amarnath, S.; Fowler, D.H.; Roberts, D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012, 287, 4211–4221.
- Isenberg, J.S.; Maxhimer, J.B.; Hyodo, F.; Pendrak, M.L.; Ridnour, L.A.; DeGraff, W.G.; Tsokos, M.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am. J. Pathol. 2008, 173, 1100–1112.
- Soto-Pantoja, D.R.; Terabe, M.; Ghosh, A.; Ridnour, L.A.; DeGraff, W.G.; Wink, D.A.; Berzofsky, J.A.; Roberts, D.D. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014, 74, 6771–6783.
- Schwartz, A.L.; Nath, P.R.; Allgauer, M.; Lessey-Morillon, E.C.; Sipes, J.M.; Ridnour, L.A.; Morillon Ii, Y.M.; Yu, Z.; Restifo, N.P.; Roberts, D.D. Antisense targeting of CD47 enhances human cytotoxic T-cell activity and increases survival of mice bearing B16 melanoma when combined with anti-CTLA4 and tumor irradiation. Cancer Immunol. Immunother. 2019, 68, 1805–1817.
- Schuepp, B.J.; Jungi, T.W. Thrombospondin-exposed human monocytes display augmented luminol-enhanced chemiluminescence upon receptor triggering. Biochem. Biophys. Res. Commun. 1991, 177, 1087–1094.
- Suchard, S.J.; Boxer, L.A.; Dixit, V.M. Activation of human neutrophils increases thrombospondin receptor expression. J. Immunol. 1991, 147, 651–659.
- Pierson, B.A.; Gupta, K.; Hu, W.-S.; Miller, J.S. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor β-1 and independent accessory cell-derived contact and soluble factors. Blood 1996, 87, 180–189.
- Engelbertsen, D.; Autio, A.; Verwilligen, R.A.F.; Depuydt, M.A.C.; Newton, G.; Rattik, S.; Levinsohn, E.; Saggu, G.; Jarolim, P.; Wang, H.; et al. Increased lymphocyte activation and atherosclerosis in CD47-deficient mice. Sci. Rep. 2019, 9, 10608.
- Doyen, V.; Rubio, M.; Braun, D.; Nakajima, T.; Abe, J.; Saito, H.; Delespesse, G.; Sarfati, M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 2003, 198, 1277–1283.
- Krispin, A.; Bledi, Y.; Atallah, M.; Trahtemberg, U.; Verbovetski, I.; Nahari, E.; Zelig, O.; Linial, M.; Mevorach, D. Apoptotic cell thrombospondin-1 and heparin binding domain lead to dendritic cell phagocytic and tolerizing states. Blood 2006, 108, 3580–3589.
- Tabib, A.; Krispin, A.; Trahtemberg, U.; Verbovetski, I.; Lebendiker, M.; Danieli, T.; Mevorach, D. Thrombospondin-1-N-terminal domain induces a phagocytic state and thrombospondin-1-C-terminal domain induces a tolerizing phenotype in dendritic cells. PLoS ONE 2009, 4, e6840.
- Mittal, R.; Gonzalez-Gomez, I.; Prasadarao, N.V. Escherichia coli K1 promotes the ligation of CD47 with thrombospondin-1 to prevent the maturation of dendritic cells in the pathogenesis of neonatal meningitis. J. Immunol. 2010, 185, 2998–3006.
- Bandyopadhyay, G.; Bandyopadhyay, S.; Bankey, P.E.; Miller-Graziano, C.L. Elevated postinjury thrombospondin 1-CD47 triggering aids differentiation of patients’ defective inflammatory CD1a+dendritic cells. J. Leukoc. Biol. 2014, 96, 797–807.
- Li, S.S.; Forslow, A.; Sundqvist, K.G. Autocrine regulation of T cell motility by calreticulin-thrombospondin-1 interaction. J. Immunol. 2005, 174, 654–661.
- Mansfield, P.J.; Boxer, L.A.; Suchard, S.J. Thrombospondin stimulates motility of human neutrophils. J. Cell Biol. 1990, 111, 3077–3086.
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092.
- Fang, L.L.; Yu, H.Q.; Wu, R.J.; He, C.; Li, M.; Yan, H.; Li, J.J.; Wang, S.; Liu, Z.G.; Liu, Z.J.; et al. Thrombospondin 1 Modulates Monocyte Properties to Suppress Intestinal Mucosal Inflammation. J. Innate Immun. 2015, 7, 601–611.
- Kuznetsova, S.A.; Issa, P.; Perruccio, E.M.; Zeng, B.; Sipes, J.M.; Ward, Y.; Seyfried, N.T.; Fielder, H.L.; Day, A.J.; Wight, T.N.; et al. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J. Cell Sci. 2006, 119, 4499–4509.
- Martin-Manso, G.; Galli, S.; Ridnour, L.A.; Tsokos, M.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity by differentiated U937 cells. Cancer Res. 2008, 68, 7090–7099.
- Catena, R.; Bhattacharya, N.; El Rayes, T.; Wang, S.; Choi, H.; Gao, D.; Ryu, S.; Joshi, N.; Bielenberg, D.; Lee, S.B.; et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013, 3, 578–589.
- Chauhan, S.; Danielson, S.; Clements, V.; Edwards, N.; Ostrand-Rosenberg, S.; Fenselau, C. Surface Glycoproteins of Exosomes Shed by Myeloid-Derived Suppressor Cells Contribute to Function. J. Proteome Res. 2017, 16, 238–246.
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930–942.
- Kim, M.J.; Lee, J.C.; Lee, J.J.; Kim, S.; Lee, S.G.; Park, S.W.; Sung, M.W.; Heo, D.S. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumor Biol. 2008, 29, 28–34.
- Jaiswal, S.; Chao, M.P.; Majeti, R.; Weissman, I.L. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010, 31, 212–219.
- Poels, L.G.; Peters, D.; van Megen, Y.; Vooijs, G.P.; Verheyen, R.N.; Willemen, A.; van Niekerk, C.C.; Jap, P.H.; Mungyer, G.; Kenemans, P. Monoclonal antibody against human ovarian tumor-associated antigens. J. Natl. Cancer Inst. 1986, 76, 781–791.
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011, 71, 1374–1384.
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010, 142, 699–713.
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y.; et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94.
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J., Jr.; Chang, H.Y.; van de Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021.
- Edris, B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Liu, J.; Majeti, R.; West, R.B.; Fletcher, J.A.; et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 6656–6661.
- Kim, D.; Wang, J.; Willingham, S.B.; Martin, R.; Wernig, G.; Weissman, I.L. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012, 26, 2538–2545.
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667.
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164.
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108.
- Zhao, X.W.; van Beek, E.M.; Schornagel, K.; Van der Maaden, H.; Van Houdt, M.; Otten, M.A.; Finetti, P.; Van Egmond, M.; Matozaki, T.; Kraal, G.; et al. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc. Natl. Acad. Sci. USA 2011, 108, 18342–18347.
- Maxhimer, J.B.; Soto-Pantoja, D.R.; Ridnour, L.A.; Shih, H.B.; DeGraff, W.G.; Tsokos, M.; Wink, D.A.; Isenberg, J.S.; Roberts, D.D. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci. Transl. Med. 2009, 1, 3ra7.
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.X.; Xu, M.M. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 2015.
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654.
- Soto-Pantoja, D.R.; Miller, T.W.; Frazier, W.A.; Roberts, D.D. Inhibitory signaling through signal regulatory protein-alpha is not sufficient to explain the antitumor activities of CD47 antibodies. Proc. Natl. Acad. Sci. USA 2012, 109, E2842.
- Kaur, S.; Cicalese, K.V.; Bannerjee, R.; Roberts, D.D. Preclinical and Clinical Development of Therapeutic Antibodies Targeting Functions of CD47 in the Tumor Microenvironment. Antib. Ther. 2020, 3, 179–192.
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients With Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953.
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721.
- Johnson, L.D.S.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C.; et al. Targeting CD47 in Sezary syndrome with SIRPalphaFc. Blood Adv. 2019, 3, 1145–1153.
- Kauder, S.E.; Kuo, T.C.; Harrabi, O.; Chen, A.; Sangalang, E.; Doyle, L.; Rocha, S.S.; Bollini, S.; Han, B.; Sim, J.; et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE 2018, 13, e0201832.
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054.
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D., Jr.; van Rooijen, N.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299.
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232.
- Manna, P.P.; Dimitry, J.; Oldenborg, P.A.; Frazier, W.A. CD47 augments Fas/CD95-mediated apoptosis. J. Biol. Chem. 2005, 280, 29637–29644.
- Manna, P.P.; Frazier, W.A. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004, 64, 1026–1036.
- Zhao, X.W.; Matlung, H.L.; Kuijpers, T.W.; van den Berg, T.K. On the mechanism of CD47 targeting in cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E2843.
- Cioffi, M.; Trabulo, S.; Hidalgo, M.; Costello, E.; Greenhalf, W.; Erkan, M.; Kleeff, J.; Sainz, B., Jr.; Heeschen, C. Inhibition of CD47 Effectively Targets Pancreatic Cancer Stem Cells via Dual Mechanisms. Clin. Cancer Res. 2015, 21, 2325–2337.
- Stein, E.V.; Miller, T.W.; Ivins-O’Keefe, K.; Kaur, S.; Roberts, D.D. Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1 beta Production and Activation through CD47. Sci. Rep. 2016, 6.
- Legrand, N.; Huntington, N.D.; Nagasawa, M.; Bakker, A.Q.; Schotte, R.; Strick-Marchand, H.; de Geus, S.J.; Pouw, S.M.; Bohne, M.; Voordouw, A.; et al. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 13224–13229.
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Jang, M.K.; Alawi, M.; Saygi, C.; Gravina, A.; Tediashvili, G.; Nguyen, V.Q.; Liu, Y.; et al. The SIRPalpha-CD47 immune checkpoint in NK cells. J. Exp. Med. 2021, 218.
- Yanagita, T.; Murata, Y.; Tanaka, D.; Motegi, S.I.; Arai, E.; Daniwijaya, E.W.; Hazama, D.; Washio, K.; Saito, Y.; Kotani, T.; et al. Anti-SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2017, 2, e89140.
- Nath, P.R.; Gangaplara, A.; Pal-Nath, D.; Mandal, A.; Maric, D.; Sipes, J.M.; Cam, M.; Shevach, E.M.; Roberts, D.D. CD47 expression in natural killer cells regulates homeostasis and modulates immune response to lymphocytic choriomeningitis virus. Front. Immunol. 2018, 9, 2985.
- Nath, P.R.; Pal-Nath, D.; Mandal, A.; Cam, M.; Schwartz, A.L.; Roberts, D.D. CD47 in the tumor microenvironment and CD47 antibody blockade regulate natural killer cell recruitment and activation. Cancer Immunol. Res. 2019, 7, 1547–1561.
- Balint, S.; Muller, S.; Fischer, R.; Kessler, B.M.; Harkiolaki, M.; Valitutti, S.; Dustin, M.L. Supramolecular attack particles are autonomous killing entities released from cytotoxic T cells. Science 2020, 368, 897–901.
- Ambrose, A.R.; Hazime, K.S.; Worboys, J.D.; Niembro-Vivanco, O.; Davis, D.M. Synaptic secretion from human natural killer cells is diverse and includes supramolecular attack particles. Proc. Natl. Acad. Sci. USA 2020, 117, 23717–23720.
- Thomas Hawighorst; Hajimu Oura; Michael Streit; Lauren Janes; Lynh Nguyen; Lawrence F Brown; Guillermo Oliver; David G Jackson; Michael Detmar; Thrombospondin-1 selectively inhibits early-stage carcinogenesis and angiogenesis but not tumor lymphangiogenesis and lymphatic metastasis in transgenic mice. Oncogene 2002, 21, 7945-7956, 10.1038/sj.onc.1205956.
- Salida Mirzoeva; Xin Tong; Bryan B. Bridgeman; Michael Plebanek; Olga V. Volpert; Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930-942, 10.1016/j.neo.2018.07.005.
- Juan Carlos Rodríguez-Manzaneque; Timothy F. Lane; María Asunción Ortega; Richard O. Hynes; Jack Lawler; M. Luisa Iruela-Arispe; Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proceedings of the National Academy of Sciences 2001, 98, 12485-12490, 10.1073/pnas.171460498.
- Bethany A. Kerr; Koran S. Harris; Lihong Shi; Jeffrey S. Willey; David R. Soto-Pantoja; Tatiana V. Byzova; Platelet TSP-1 Controls Prostate Cancer-Induced Osteoclast Differentiation and Bone Marrow-Derived Cell Mobilization through TGFβ-1. bioRxiv 2020, 2020, 2020.2002.2011.943860, 10.1101/2020.02.11.943860.
- Linda S. Gutierrez; Mark Suckow; Jack Lawler; Victoria A. Ploplis; Francis J. Castellino; Thrombospondin 1--a regulator of adenoma growth and carcinoma progression in the APCMin/+ mouse model. Carcinogenesis 2003, 24, 199-207, 10.1093/carcin/24.2.199.
- Zenaida P. Lopez-Dee; Sridar V. Chittur; Hiral Patel; Aleona Chinikaylo; Brittany Lippert; Bhumi Patel; Jack Lawler; Linda S. Gutierrez; Thrombospondin-1 in a Murine Model of Colorectal Carcinogenesis. PLOS ONE 2015, 10, e0139918, 10.1371/journal.pone.0139918.
- D R Soto-Pantoja; J M Sipes; G Martin-Manso; B Westwood; N L Morris; A Ghosh; N J Emenaker; D D Roberts; Dietary fat overcomes the protective activity of thrombospondin-1 signaling in the Apc(Min/+) model of colon cancer.. Oncogenesis 2016, 5, e230-e230, 10.1038/oncsis.2016.37.
- Manuel U. Ramirez; Elizabeth R. Stirling; Nancy J. Emenaker; David D. Roberts; David R. Soto-Pantoja; Thrombospondin-1 interactions regulate eicosanoid metabolism and signaling in cancer-related inflammation. Cancer and Metastasis Reviews 2018, 37, 469-476, 10.1007/s10555-018-9737-x.
- Scot Ebbinghaus; Maha Hussain; Nizar Tannir; Michael Gordon; Apurva A. Desai; Raymond A. Knight; Rod A. Humerickhouse; Jiang Qian; Gary B. Gordon; Robert Figlin; et al. Phase 2 Study of ABT-510 in Patients with Previously Untreated Advanced Renal Cell Carcinoma. Clinical Cancer Research 2007, 13, 6689-6695, 10.1158/1078-0432.ccr-07-1477.
- Svetomir N. Markovic; Vera J. Suman; Ravi A. Rao; James N. Ingle; Judith S. Kaur; Lori A. Erickson; Henry C. Pitot; Gary A. Croghan; Robert R. McWilliams; Jaime Merchan; et al. A Phase II Study of ABT-510 (Thrombospondin-1 Analog) for the Treatment of Metastatic Melanoma. American Journal of Clinical Oncology 2007, 30, 303-309, 10.1097/01.coc.0000256104.80089.35.
- Laurence H. Baker; Eric K. Rowinsky; David Mendelson; Rod A. Humerickhouse; Raymond A. Knight; Jiang Qian; Robert A. Carr; Gary B. Gordon; George D. Demetri; Randomized, Phase II Study of the Thrombospondin-1-Mimetic Angiogenesis Inhibitor ABT-510 in Patients With Advanced Soft Tissue Sarcoma. Journal of Clinical Oncology 2008, 26, 5583-5588, 10.1200/jco.2008.17.4706.
- Thomas W. Miller; Joshua D. Amason; Elsa Garcin; Laurence Lamy; Patricia K. Dranchak; Ryan MacArthur; John Braisted; Jeffrey S. Rubin; Teresa L. Burgess; Catherine L. Farrell; et al. Quantitative high-throughput screening assays for the discovery and development of SIRPα-CD47 interaction inhibitors. PLOS ONE 2019, 14, e0218897, 10.1371/journal.pone.0218897.
