Non-mechanical hybrid hydrogen compressors consists of a first electrochemical compression stage followed by a second one based on the adsorption-desorption of hydrogen on microporous materials. They allow compressing hydrogen up to 70 MPa. Non-mechanical hybrid hydrogen compressors can be a valid alternative to the mechanical compressors.
- Hydrogen storage
- Hydrogen compression
- Electrochemical compressors
- Hydrogen adsorption
- Activated carbons
1. Definition
Non-mechanical hydrogen compressors have proven to be a valid alternative to mechanical compressors. Among these, electrochemical compressors allow isothermal, and therefore highly efficient, compression of hydrogen. On the other hand, adsorption-desorption compressors allow hydrogen to be compressed through cooling/heating cycles using highly microporous materials as hydrogen adsorbents. A non-mechanical hybrid hydrogen compressor, consisting of a first electrochemical stage followed by a second stage driven by adsorption-desorption of hydrogen on activated carbons, allows hydrogen to be produced at 70 MPa, a value currently required for the development of hydrogen automotive applications. This system has several advantages over mechanical compressors, such as the absence of moving parts and high compactness. Its use in decentralized hydrogen facilities, such as hydrogen refueling stations, can be considered.
2. Introduction
Since hydrogen is widely used in industry for the production of ammonia and the hydrogenation of petroleum products, the hydrogen sector, which includes production in decentralized facilities, storage and distribution, is already mature. However, the potential benefits of hydrogen as a fuel can be realized once storage methods are optimized and an efficient and safe distribution infrastructure is in place. In this context, the storage of hydrogen requires its compression. Mechanical compressors (piston, diaphragm, linear, and ionic liquid compressors), which are universally used for the compression of all gases, are not very suitable for the specific case of hydrogen. Research on new compression technologies, such as non-mechanical hydrogen compressors (metal hydrides, electrochemical, and adsorption-desorption compressors) is thus highly demanded, particularly with regard to the development of decentralized infrastructure for the production and use of hydrogen in situ.
3. Data, Model, Applications and Influences
3.1. Non-Mechanical Hydrogen Compressors
3.1.1. Metal Hydride Compressors
3.1.2. Electrochemical Compressors
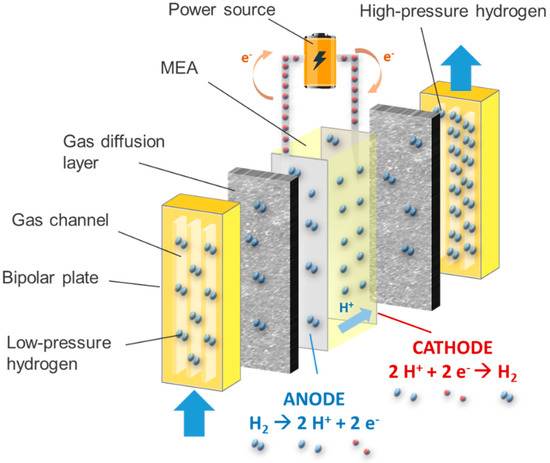
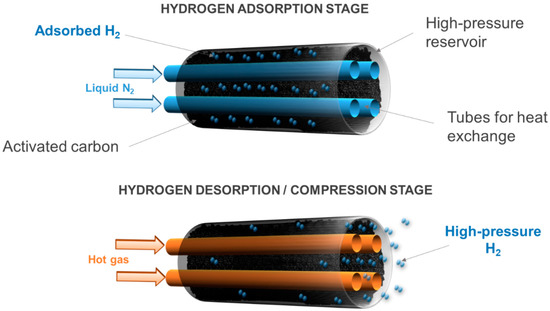
3.1.3. Adsorption–Desorption Compressors
4. Conclusions
Reducing the cost of hydrogen storage is crucial for the development of automotive hydrogen applications, such as fuel cell vehicles. In fact, the storage, transportation, and distribution stages cause significant increases in the price of hydrogen at the pump, which is currently at USD 8–10 kg−1.
High-pressure hydrogen storage has been proven to be the most suitable method for storing hydrogen in decentralized facilities, i.e., at hydrogen refueling stations, compared to liquid-phase storage and storage in absorbed form in solid materials. Nevertheless, mechanical compressors, which are the most widely used technology for compressing hydrogen today, are responsible for more than 50% of CAPEX, 20% of OPEX, and about 30% of the total energy consumption of a hydrogen refueling station. Furthermore, mechanical compressors have several disadvantages, such as the presence of many moving parts, hydrogen embrittlement, high consumption of energy, high structural complexity, and difficult heat management.
Non-mechanical compressors, such as metal hydride, electrochemical, and adsorption–desorption compressors, may be a suitable alternative to replace mechanical compressors in decentralized facilities. Indeed, they have several advantages, such as the absence of moving parts that contributes significantly to the reduction of installation and maintenance costs compared to mechanical compressors. Metal hydride compressors ensure both safe storage and compression of hydrogen. As they require heat exchange, they are also known as thermally driven compressors. The search for appropriate alloys is essential for the development of such technology, as it requires both low desorption temperatures and high pressures.
Electrochemical compressors are based on the use of selective polymeric membranes, such as Nafion®, to compress hydrogen gas, and have been proven to provide the highest level of compression efficiency (up to 60%) when the discharge pressure is not too high. Indeed, the efficiency of such devices is affected by the hydrogen back-diffusion. Electrochemical compressors have been found to offer good performances when equipped with a thin membrane at 1 A cm−2.
Adsorption–desorption compressors rely on the ability of hydrogen to bind weakly to the surface of highly porous solids, such as carbon materials or metal-organic frameworks. Like metal hydride compressors, adsorption–desorption compressors are also thermally driven. Nevertheless, operation at a cryogenic temperature, down to 77 K, is required to enhance hydrogen uptakes. Such devices are able to compress hydrogen up to 70 MPa in a single step.
Hybrid configurations, consisting of: (i) a first electrochemical stage up to 4–8 MPa; and (ii) a second stage based on cyclic adsorption–desorption on carbon materials, make it possible to reach 70 MPa in a compact and quiet device. This could represent a promising alternative to mechanical hydrogen compressors in the framework of decentralized facilities, such as hydrogen refueling stations.
References
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Metal hydride hydrogen compressors: A review. Int. J. Hydrog. Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, H. A 70 MPa hydrogen-compression system using metal hydrides. Int. J. Hydrog. Energy 2011, 36, 9079–9085. [Google Scholar] [CrossRef]
- Stamatakis, E.; Zoulias, E.; Tzamalis, G.; Massina, Z.; Analytis, V.; Christodoulou, C.; Stubos, A. Metal hydride hydrogen compressors: Current developments & early markets. Renew. Energy 2018, 127, 850–862. [Google Scholar] [CrossRef]
- Moton, J.M.; James, B.D.; Colella, W.G. Advances in Electrochemical Compression of Hydrogen. In Proceedings of the ASME 2014 12th International Conference on Fuel Cell Science, Engineering and Technology, Boston, MA, USA, 30 June–2 July 2014. [Google Scholar]
- Trégaro, M.; Rhandi, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part II. Challenges in electrocatalysis. Chin. J. Catal. 2020, 41, 770–782. [Google Scholar] [CrossRef]
- Ströbel, R.; Oszcipok, M.; Fasil, M.; Rohland, B.; Jörissen, L.; Garche, J. The compression of hydrogen in an electrochemical cell based on a PE fuel cell design. J. Power Sources 2002, 105, 208–215. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Pasierb, P.; Rekas, M. High-Temperature Electrochemical Hydrogen Pumps and Separators. Int. J. Electrochem. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Suermann, M.; Kiupel, T.; Schmidt, T.J.; Büchi, F.N. Electrochemical Hydrogen Compression: Efficient Pressurization Concept Derived from an Energetic Evaluation. J. Electrochem. Soc. 2017, 164, F1187–F1195. [Google Scholar] [CrossRef]
- Sdanghi, G.; Dillet, J.; Didierjean, S.; Fierro, V.; Maranzana, G. Feasibility of Hydrogen Compression in an Electrochemical System: Focus on Water Transport Mechanisms. Fuel Cells 2019, 20, 370–380. [Google Scholar] [CrossRef]
- HyET. Hydrogen Efficiency Technologies. Available online: http://www.hyet.nl/newsite/technology/working-principle (accessed on 15 March 2017).
- Rohland, B.; Eberle, K.; Ströbel, R.; Scholta, J.; Garche, J. Electrochemical hydrogen compressor. Electrochim. Acta 1998, 43, 3841–3846. [Google Scholar] [CrossRef]
- Wiebe, W.; Unwerth, T.V.; Schmitz, S. Hydrogen pump for hydrogen recirculation in fuel cell vehicles. E3S Web Conf. 2020, 155, 01001. [Google Scholar] [CrossRef]
- Tao, Y.; Lee, H.; Hwang, Y.; Radermacher, R.; Wang, C. Electrochemical compressor driven metal hydride heat pump. Int. J. Refrig. 2015, 60, 278–288. [Google Scholar] [CrossRef]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A 70 MPa hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Carbon 2020, 161, 466–478. [Google Scholar] [CrossRef]
- Pierre, M.; Tapan, B. L’hydrogène; John Libbey Eurotext: Arcueil, France, 2006; ISBN 978-2-7420-1318-0. [Google Scholar]
- Rhandi, M.; Trégaro, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part I. Pros and cons. Chin. J. Catal. 2020, 41, 756–769. [Google Scholar] [CrossRef]
- Kadono, K.; Kajiura, H.; Shiraishi, M. Dense hydrogen adsorption on carbon subnanopores at 77 K. Appl. Phys. Lett. 2003, 83, 3392–3394. [Google Scholar] [CrossRef]
- Poirier, E.; Dailly, A. On the Nature of the Adsorbed Hydrogen Phase in Microporous Metal−Organic Frameworks at Supercritical Temperatures. Langmuir 2009, 25, 12169–12176. [Google Scholar] [CrossRef] [PubMed]
- Ting, V.P.; Ramirez-Cuesta, A.J.; Bimbo, N.; Sharpe, J.E.; Noguera-Diaz, A.; Presser, V.; Rudic, S.; Mays, T.J. Direct Evidence for Solid-like Hydrogen in a Nanoporous Carbon Hydrogen Storage Material at Supercritical Temperatures. ACS Nano 2015, 9, 8249–8254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, H.; Bénard, P.; Chahine, R. Numerical study of thermal effects in cryo-adsorptive hydrogen storage tank. J. Renew. Sustain. Energy 2013, 5, 021414. [Google Scholar] [CrossRef]
- Wang, L.W.; Tamainot-Telto, Z.; Thorpe, R.; Critoph, R.E.; Metcalf, S.J.; Wang, R.Z. Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration. Renew. Energy 2011, 36, 2062–2066. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Metal hydride hydrogen compressors: A review. Int. J. Hydrog. Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, H. A 70 MPa hydrogen-compression system using metal hydrides. Int. J. Hydrog. Energy 2011, 36, 9079–9085. [Google Scholar] [CrossRef]
- Stamatakis, E.; Zoulias, E.; Tzamalis, G.; Massina, Z.; Analytis, V.; Christodoulou, C.; Stubos, A. Metal hydride hydrogen compressors: Current developments & early markets. Renew. Energy 2018, 127, 850–862. [Google Scholar] [CrossRef]
- Moton, J.M.; James, B.D.; Colella, W.G. Advances in Electrochemical Compression of Hydrogen. In Proceedings of the ASME 2014 12th International Conference on Fuel Cell Science, Engineering and Technology, Boston, MA, USA, 30 June–2 July 2014. [Google Scholar]
- Trégaro, M.; Rhandi, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part II. Challenges in electrocatalysis. Chin. J. Catal. 2020, 41, 770–782. [Google Scholar] [CrossRef]
- Ströbel, R.; Oszcipok, M.; Fasil, M.; Rohland, B.; Jörissen, L.; Garche, J. The compression of hydrogen in an electrochemical cell based on a PE fuel cell design. J. Power Sources 2002, 105, 208–215. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Pasierb, P.; Rekas, M. High-Temperature Electrochemical Hydrogen Pumps and Separators. Int. J. Electrochem. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Suermann, M.; Kiupel, T.; Schmidt, T.J.; Büchi, F.N. Electrochemical Hydrogen Compression: Efficient Pressurization Concept Derived from an Energetic Evaluation. J. Electrochem. Soc. 2017, 164, F1187–F1195. [Google Scholar] [CrossRef]
- Sdanghi, G.; Dillet, J.; Didierjean, S.; Fierro, V.; Maranzana, G. Feasibility of Hydrogen Compression in an Electrochemical System: Focus on Water Transport Mechanisms. Fuel Cells 2019, 20, 370–380. [Google Scholar] [CrossRef]
- HyET. Hydrogen Efficiency Technologies. Available online: http://www.hyet.nl/newsite/technology/working-principle (accessed on 15 March 2017).
- Rohland, B.; Eberle, K.; Ströbel, R.; Scholta, J.; Garche, J. Electrochemical hydrogen compressor. Electrochim. Acta 1998, 43, 3841–3846. [Google Scholar] [CrossRef]
- Wiebe, W.; Unwerth, T.V.; Schmitz, S. Hydrogen pump for hydrogen recirculation in fuel cell vehicles. E3S Web Conf. 2020, 155, 01001. [Google Scholar] [CrossRef]
- Tao, Y.; Lee, H.; Hwang, Y.; Radermacher, R.; Wang, C. Electrochemical compressor driven metal hydride heat pump. Int. J. Refrig. 2015, 60, 278–288. [Google Scholar] [CrossRef]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A 70 MPa hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Carbon 2020, 161, 466–478. [Google Scholar] [CrossRef]
- Pierre, M.; Tapan, B. L’hydrogène; John Libbey Eurotext: Arcueil, France, 2006; ISBN 978-2-7420-1318-0. [Google Scholar]
- Rhandi, M.; Trégaro, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part I. Pros and cons. Chin. J. Catal. 2020, 41, 756–769. [Google Scholar] [CrossRef]
- Kadono, K.; Kajiura, H.; Shiraishi, M. Dense hydrogen adsorption on carbon subnanopores at 77 K. Appl. Phys. Lett. 2003, 83, 3392–3394. [Google Scholar] [CrossRef]
- Poirier, E.; Dailly, A. On the Nature of the Adsorbed Hydrogen Phase in Microporous Metal−Organic Frameworks at Supercritical Temperatures. Langmuir 2009, 25, 12169–12176. [Google Scholar] [CrossRef] [PubMed]
- Ting, V.P.; Ramirez-Cuesta, A.J.; Bimbo, N.; Sharpe, J.E.; Noguera-Diaz, A.; Presser, V.; Rudic, S.; Mays, T.J. Direct Evidence for Solid-like Hydrogen in a Nanoporous Carbon Hydrogen Storage Material at Supercritical Temperatures. ACS Nano 2015, 9, 8249–8254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, H.; Bénard, P.; Chahine, R. Numerical study of thermal effects in cryo-adsorptive hydrogen storage tank. J. Renew. Sustain. Energy 2013, 5, 021414. [Google Scholar] [CrossRef]
- Wang, L.W.; Tamainot-Telto, Z.; Thorpe, R.; Critoph, R.E.; Metcalf, S.J.; Wang, R.Z. Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration. Renew. Energy 2011, 36, 2062–2066. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/en13123145
