Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cognitive impairment is frequent in pediatric cancer, and behavioral and psychological disturbances often also affect children who have survived cancer problems. Furthermore, pediatric tumors are also often associated with sleep disorders.
- neurodevelopmental disorders
- intellectual disability
- neurocognitive deficit
- sleep
- cancer
- tumor
1. Introduction
Cancer is the leading cause of disease-related death among children, although recent therapeutic advances have improved life expectancy; in fact, nearly 90% of children diagnosed with cancer survive at least five years after diagnosis and over 70% at ten years [1].
The most common tumors diagnosed in childhood are leukemias (acute lymphoblastic leukemia in 78% of cases), brain neoplasms (especially astrocytoma and non-Hodgkin’s lymphoma) and of the central nervous system (CNS), which represent 34%, 23% and 12% of all childhood cancers, respectively. Solid tumors that are not localized in the CNS are very rare. Age strongly influences cancer occurring in childhood: the highest incidence is in the age group under five years (characterized by rapid brain and functional development and, therefore, a period in which the brain is particularly vulnerable), and a second peak of incidence occurs during puberty [2].
In recent years, a close relationship between pediatric cancer and cognitive impairment has been established, as well as behavioral and psychological disturbances in children who have survived cancer problems [3,4,5,6,7]; it has been estimated that 40 to 100% of brain tumor survivors have neurocognitive problems [8]. Cancer treatment can also impact negatively on the child’s health, even after recovery [3,7,9,10].
Furthermore, several studies have been conducted concerning the association between pediatric tumors and sleep disorders, highlighting how these are frequent in children with malignancies [2,11,12], but there is a scarcity of data on the neurodevelopmental effects associated with both cancer and sleep in children. The aim of this narrative is therefore to shed light on the correlation between sleep disorders, neurodevelopmental disorders and pediatric neoplasms in children.
Data for this review consisted of empirical articles published in peer-reviewed journals between 2010 and February 2020. An extensive computer-assisted literature search was conducted using PubMed, selecting only studies involving humans. Several searches were carried out using the following terms in various combinations: “pediatric cancer”, “neurodevelopment”, “pediatric tumor”, “neurodevelopmental disorder”, “sleep”, “leukemia”, “pediatric leukemia”, and “adolescent cancer”. The search for the association between specific sleep disorders (insomnia, parasomnia, hypersomnia, narcolepsy, sleepwalking, night terrors, restless legs syndrome, periodic limb movement, somnambulism) and “pediatric cancer”, “tumor”, and “neurodevelopment” did not retrieve any records.
Excluded from consideration were book chapters, monographs, commentaries, review articles, dissertations, abstracts, letters to the editor, and any non-data-analytic or non-peer-reviewed reports. Duplicates were also excluded, and the reference list of the retrieved articles was reviewed and pertinent articles published during the same time period specified above were added to the final list of papers. However, only four articles [13,14,15,16] dealt with cancer, neurodevelopmental disorders and sleep at the same time.
1.1. Pediatric Cancer as a Cause of Neurodevelopmental Disorders
The influence of pediatric tumors and their treatment on neural development and on behavioral and emotional outcomes, even in the long term, has only recently been investigated [3,4,5,6,7]. It has been reported that cancer survivors treated with radio-chemotherapy show a high rate of cognitive dysfunction, with attention deficits in 67% and memory deficits in 3–28% of cases, as well as disorders of executive functions and speed of elaboration [17,18].
The onset of psychological problems (such as post-traumatic stress disorder) is very frequent, which can negatively impact on school performance and daily performance, as well as determine a reduction in therapeutic adherence, with a consequent increase in morbidity and mortality [19].
The onset of post-traumatic stress disorder may be due both to the diagnosis of malignant disease (resulting in a psychological impact on the child and the family) and to the anti-cancer treatment [20]. The management of the psychological sphere in oncology, therefore, plays a predominant role and, considering the close correlation between sleep disorders and psychiatric and neurodevelopment problems [21], treating sleep disorders can also be very useful in the treatment of cancer, being able to positively influence the psychological and cognitive components. In recent years, it has in fact been shown that sleep deprivation can affect neurodevelopment in children and adolescents, with repercussions on physical and mental health also causing structural alterations of the brain circuits in the frontal and limbic region, involved in the circuits of emotion [21,22].
The neurobiological changes observed are largely attributed to the neurotoxic effects of anticancer treatments [7], with dose-dependent effects of these therapies on brain structure and function [23,24,25]; these harmful effects on neurodevelopment also occur in the case of therapies for the treatment of leukemia [3], solid CNS tumors [10], and solid tumors not localized in the CNS [9,26].
In the case of brain tumors, in addition to radio-chemotherapy treatment, the localization of the tumor can also affect the neurocognitive outcome, with infratentorial tumors associated with worse outcomes compared to those with a higher localization: a study compared verbal and non-verbal intellectual functions, working, visual and verbal memory, visual–spatial integration, attention, and social and emotional functions in two groups of children with brain tumors (subjected to resection of the tumor and at the same doses of radiotherapy), and taking into account the localization of the brain tumor. Children with infratentorial tumors had lower school scores (and a greater frequency of hearing deficits) than children with supratentorial cancer [27].
Cognitive and psychological problems related to the onset of cancer can also be influenced by the age of the child at the time of diagnosis [28] and by the timing with which radio-chemotherapy treatment is carried out, which is more harmful in the case of early treatment [10] and capable of damaging several subcortical regions involved in the integration of affective and motivationally relevant signals, such as the amygdala, the thalamus, the ventral striatum, the substantia nigra/ventral tegmental area and, finally, the hippocampus, which plays a key role in the circuits of memory and emotions connected to the aforementioned structures [29].
The brain in the first years of life is particularly vulnerable to the negative effects of treatment due to rapid cell proliferation, dendritic and axonal growth, and myelination, which occur in childhood and adolescence [10]. The maturation of the gray and white matter is damaged, with a consequent slower cognitive processing speed, as the glial progenitor cells (responsible for the formation of oligodendrocytes and astrocytes) and hippocampal cells (involved in the processes of neurogenesis) are particularly vulnerable to the effects of chemo and radiotherapy [30], both in patients with leukemia [3], and in patients with solid CNS tumors [10].
The damage is due to both direct cell toxicity, induced by chemotherapy, and to inflammation and oxidative stress (i.e., indirect toxicity), which seem to have a negative impact especially on the hippocampus and prefrontal regions, causing behavioral disorders, such as lack of self-control before and during adolescence [31,32].
As mentioned above, acute lymphoblastic leukemia (ALL), the form of neoplasm that occurs most frequently in children, is a cause of impaired neurodevelopment of the child [3,33], but only in recent years have the specific biological mechanisms acting on long-term neuronal integrity, induced by ALL and the therapies administered for this pathology, been specified.
A recent study evaluated patients aged between 8 and 21 years treated with a single chemotherapy protocol with methotrexate and demonstrated a brain connectome dysfunction; the results were consistent with a delay in neurodevelopment (especially in younger children), which could be associated with reduced recovery capacity, adaptability and flexibility of the brain network [34].
Functional magnetic resonance imaging studies conducted in children with ALL have also shown worse neurocognitive dysfunction in the case of early treatment and with a higher dosage of methotrexate, highlighting a reduction in the activity of the right temporal lobe and of the frontal and parietal lobes, bilaterally [35].
However, in children with ALL, the white matter seems to be damaged even before chemotherapy treatment [13]. A very interesting study also evaluated cerebrospinal fluid biomarkers, demonstrating a cytokine-mediated inflammatory mechanism that, once it passes the blood–brain barrier, may trigger a cascade of neurotoxic events.
An increase in tau protein was observed (suggestive of axonal damage) in association with a worsening of attention deficit and a reduction in intelligence quotient; an increase in glial fibrillary acidic protein (GFAP) concentration in patients with an altered allele of the apolipoprotein E (APOE) gene (associated with deficiency of attention); and an increase in leukoencephalopathy, with compromised white matter especially in the frontal (particularly in the dopaminergic circuits) and parietal [36] lobes. This study also showed an impairment of brain structures both before and after chemotherapy: glial damage was present at the diagnosis; after intrathecal chemotherapy, neuronal damage was triggered, which was worse in cases of higher methotrexate dosage, especially in patients with the Val allele in the catechol-O-methyltransferase (COMT) gene and early treatment [36].
Table 1 summarizes the main biological mechanisms of CNS damage occurring in the course of pediatric cancer.
Table 1. Biological damage of the central nervous system observed in pediatric cancer.
- - Damage of the hippocampus and other areas involved in memory emotion circuits (amygdala, thalamus, striatum, substantia nigra/ventral tegmental area) [10,29,30,31,32]
- - Dendrite and axonal growth damage [10]
- - Increase in tau protein (axonal damage and neurodegeneration), increase in GFAP (associated with attention deficit) [36]
The relationship and interactions between pediatric cancer, chemotherapy, sleep and CNS development/damage and cognitive function are complex and schematically represented in Figure 1.
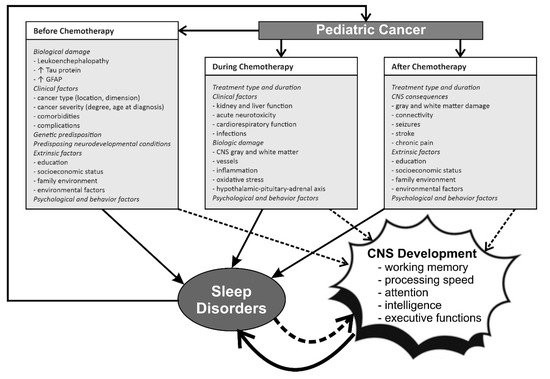
Figure 1. The complex relationship between pediatric cancer, chemotherapy, CNS development and sleep before, during, and after treatment. GFAP, glial fibrillary acidic protein. ↓ decrease; ↑ increase.
The above results highlight the need for interventions that prevent or manage cognitive impairment in pediatric ALL; in recent years, the results of cognitive behavioral therapy (CBT) and physical activity in pediatric patients with ALL or solid CNS tumors have been examined, with improvement of brain function and an increase in white matter and hippocampal volume [38,39,40]. These data underline that the treatment of psychological problems in children with cancer is also of fundamental importance because it involves a potential improvement in cognitive performance; on the other hand, it has been shown that correct sleep hygiene is very important for the protection of the physical and mental health of children and that health workers take care of this aspect in clinical practice [21].
The studies conducted on the effects of pediatric tumors not located in the CNS and their treatment on neuronal development are indeed very few (given the rarity of these tumors). However, even in this case, there seems to be evidence of damage related to neuro-inflammation and chemotherapy-induced damage of the blood–brain barrier [9,26]. Finally, in these types of tumors chemotherapy can damage the hypothalamic–pituitary–adrenal axis [37], altering hormonal structure and inducing growth problems and depressive symptoms [9].
Therefore, before or during radio-chemotherapy, an early treatment of psychological and cognitive problems seems to be appropriate in order to positively influence the maturation processes of the hippocampus and related structures and the developing neuronal plasticity [7]. It seems that the hippocampus is one of the few brain areas that shows active postnatal neurogenesis, which makes it particularly susceptible to changes induced by pediatric tumors [41].
1.2. Pediatric Tumors Arising in Comorbidity with Neurodevelopmental Disorders
Regarding the studies conducted on congenital neurological development anomalies in pediatric cancer and young adults, there are a few reports in the literature showing that 8–30% of pediatric oncology patients have mutations in cancer-predisposing genes [42].
There is a single study that expands previous reports on the associations of congenital anomalies with pediatric tumors by integrating neurocognitive deficits; this study examined cancer patients aged 0 to 23 years, identifying congenital anomalies before the onset of the tumor in 141 (13% of cases) out of a sample of 1107 patients; specifically, the following pathologies were observed: movement disorders, obstructive hydrocephalus, Arnold–Chiari malformation, spina bifida, cerebral palsy, epilepsy and, less frequently, cardiovascular, gastrointestinal or genitourinary tract anomalies. Congenital anomalies of the CNS were found to be associated with CNS tumors (less frequently with astrocytomas and ependymomas, more frequently with gliomas and neuroblastomas), especially in males aged less than 5 years [43].
A correlation between neurofibromatosis, tumors and neurodevelopmental disorders is also known; in particular, a compromise in the formation process of dendritic spines has been observed [44].
Genetic predisposition, therefore, plays a key role in this area, and should also be taken into consideration for chemotherapy treatment in these children. Indeed, recent studies have highlighted specific genetic polymorphisms connected to different alterations of cognitive performances or behavioral disorders; a role for genes related to oxidative stress and neuro-inflammation is believed to contribute to the neurocognitive decline associated with chemotherapy in children with ALL [31,45,46]. It is possible that some DNA polymorphisms related to brain development, APOE and brain-derived neurotrophic factor (BDNF) may provide protection against neurotoxicity during development; however, further studies are needed because it is not clear which genotypes and immunological mechanisms are involved in chemotherapy-induced neurotoxicity [9]. Finally, radiation therapy is suspected to induce a shortening of telomeres [47].
2. The Role of Sleep Disorders in Children with Cancer and Neurodevelopmental Disorders
The close correlation between sleep disturbances and pediatric cancer [2,11,12] and how the treatment of tumors can negatively impact children’s health, even after recovery from cancer, has recently been highlighted [3,7,9,10]; however, papers on this topic are not homogeneous and evidence is scattered around in several observational studies and case series and reports that are briefly described below.
Since pediatric brain tumor survivors are at risk of both sleep disturbance and neurocognitive impairment, improving sleep may be a way to promote neurocognitive functioning in these children, for whom adequate interventions are currently scarce [48]. It is also true that a knowledge of sleep disorders in children with neurodevelopmental pathologies predisposed to develop cancer can improve their health and quality of life; however, there are only few studies in the literature that have evaluated this aspect, highlighting a high prevalence of sleep disturbances in tuberous sclerosis (74% of cases) and in autism spectrum disorder (50–80% of cases) [49].
One of the first attempts to demonstrate that neurocognitive function (attention, memory and processing speed deficits) in long-term survivors of childhood cancer appears to be particularly vulnerable to the effects of fatigue and sleep fragmentation dates back to 2011, and suggested that sleep hygiene could be a valid tool to improve neurocognitive outcome [14].
As previously said, neoplasms in children are associated with neurodevelopmental problems related to neuroinflammation [36]; there is growing evidence that systemic inflammation is associated with poor sleep quality [50]; and sleep duration is linked to serum concentrations of C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α) and interleukin [51]. Table 2 summarizes some significant aspects of the importance of sleep for the developing brain.
Table 2. The identified roles of sleep in the development of the central nervous system and cancer.
- Improvement of neuronal plasticity [52,53]-
Immunity system potentiation [13]
- Sleep slow-wave maturation stimulates brain connectivity [51,56,57,58,59]-
Sleep deprivation causes an increase in tau protein [60]
- Melatonin
3. The Effect of Sleep Disorder Treatment on Neurodevelopmental Disorders in Children with Cancer
Studies on the treatment of sleep disorders in children with oncological problems and neurodevelopmental disorders are very few and concern the usefulness of CBT and melatonin; in the latter case, the greatest evidence is provided by studies conducted on adults [15,16,53,54,60,62,63,64]. Further studies in this regard could be very useful for improving the therapeutic outcome and quality of life in children with cancer and cognitive problems.
This entry is adapted from the peer-reviewed paper 10.3390/brainsci10070411
This entry is offline, you can click here to edit this entry!
