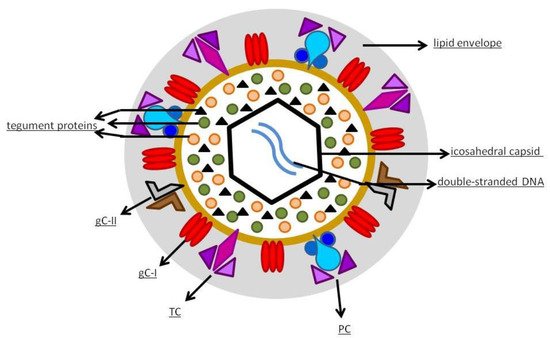
Despite the clear need, progress toward a vaccine for hCMV has been slow. The first attempts to create a hCMV vaccine began in the 1970s, when two strains of the virus, Towne and AD169, were attenuated in order to function as active immunoprophylaxis [
92,
93]. The project with the Towne strain was developed by Stanley Plotkin and provided clinical studies with SOT recipients: initial results seemed promising, but statistical analyses revealed that protection against infection was not significant [
94,
95]. Subsequently, a growing number of hCMV vaccine candidates were developed and are now at different stages, but to date, none have been licensed. There are many intrinsic features of hCMV that are challenging for the design of a vaccine. It can cause persistent asymptomatic infection and establish a lifelong latency in the host after subclinical primary infection [
85]; it is able to spread cell–cell, avoiding antibodies in the extracellular fluid [
96]; moreover, hCMV reactivations can happen during periods of decreased immune system defenses [
85]. Another critical consideration concerns interstrain diversity: hCMV undergoes pervasive recombination with disruptive mutations identified in clinical isolates [
97], even with rapid intra-host evolution [
98], so that reinfections can occur with different strains [
99]. Moreover, hCMV proliferation in the host is species restricted so there are no natural models available to test vaccine strategies [
85].
It is well established that children born with congenital infection and immunocompromised subjects are the two groups of patients suffering the most serious consequences after contact with hCMV. Thus, despite the ideal target populations still being controversial at present, it seems reasonable that the more suitable could be pregnant women or women of childbearing age and subjects undergoing transplant [
100]. Regarding the vaccination of transplant recipients, the major challenge is inducing an adequate immunity in an immunocompromised individual. It seems that both T cells and neutralizing antibodies are implicated and should be stimulated by a hCMV vaccine in this target population [
101]. Concerning cCMV, considering the epidemiological characteristics and modality of vertical transmission, the vaccine should be able to protect both seronegative women from primary infection and seropositive women from reinfection and reactivation [
100]. It has been also proposed to include the hCMV vaccine into the routine childhood vaccination schedule, supporting universal vaccination, bearing in mind that the hCMV infection implications are not yet completely clear and that many women are infected by their own children or during jobs that require close contact with children, so the vaccination of toddlers would probably provide indirect protection [
82]. With regards to cCMV, we still do not know the exact details of immune mechanisms against this virus, but most data indicate that the vaccine should stimulate both the cellular and humoral components [
102]. After a primary maternal hCMV infection in the first trimester, hyperimmunoglobulin administration apparently prevents maternal–fetal transmission, implicitly suggesting a role of antibodies [
103], and CD4+ T cells have been correlated with protection against hCMV [
104,
105]. Considering that infants born to mothers who underwent reinfection or reactivation during pregnancy are still at risk for congenital disease, and that we do not know the specific contributions of humoral and cellular immunity for the prevention of this condition, it could seem very difficult to create an effective vaccine for avoiding vertical transmission [
90]. A recent systematic review [
5], however, confirmed that the hCMV placental transmission rate is lower in non-primary than in primary infection, with low cCMV infection prevalence in highly seropositive populations. In addition, Tabata et al. [
106] showed that human monoclonal antibodies to gB and PC prevent infection in placental cells and anchoring villi better than hyperimmunoglobulin. This evidence might prove that maternal immunity confers protection and pregnant women and women of child-bearing age could be a good target for a hCMV vaccine. Efforts to produce the vaccine have focused on few antigens; neutralizing antibody targets, such as gB, gH, and PC; and T cell epitopes, such as pp65 and IE1 [
102]. Initially, gB seemed a perfect choice, but trials showed limited efficacy [
102]. At present, not only neutralization but also other antibody mechanisms are known to play a role, such as the induction of complement-mediated virus lysis and antibody-dependent cellular cytotoxicity [
102]. A very important step for the development of hCMV vaccine is represented by the identification of new viral glycoproteins and cellular receptors implicated in virus entry in host cells, as previously described [
22]. This new awareness led to investigation of the humoral response to hCMV infection, to understand which of the viral antigens could be more important to obtain protective antibodies and to be used in a vaccine [
22]. Fouts et al. studied the anti-CMV hyperimmune globulin used to prevent hCMV disease in SOT patients and cCMV and found that the most neutralizing response was provided by antibodies directed against PC, with a little role of anti-gB antibodies [
107]. Similar conclusions were also drawn from other studies [
108,
109], thus supporting the development of PC-based vaccines. To date, the most promising choice in developing hCMV vaccines seems to be an approach that requires the expression of several antigens, with epitopes able to stimulate both the humoral and the cellular components; moreover, the role of protein conformation and structural biology in elicitation of the right immune response has been recognized, as in the case of the pre- and post-fusion crystal structure of gB [
102]. Consequently, mRNA and vector vaccines, able to simultaneously synthesize a combination of antigens in their original three-dimensional conformation, could represent good candidates [
102]. Chauhan and Singh recently described an immuno-informatics approach to design a multi-epitope vaccine [
110]. In the study, a multi-epitope vaccine was constructed thanks to immuno-informatics servers, which selected the most suitable epitopes of PC, gB, and pp65. The results showed that the vaccine might be immunogenic, with high affinity with the immune receptor, effective in stimulating different immune cell types, and could provide long-lasting immune response.