1. Introduction
It has long been known that the bicuspid aortic valve (BAV) is the most common congenital heart defect, with a prevalence differing according to studies (0.5–2% of births) [
1,
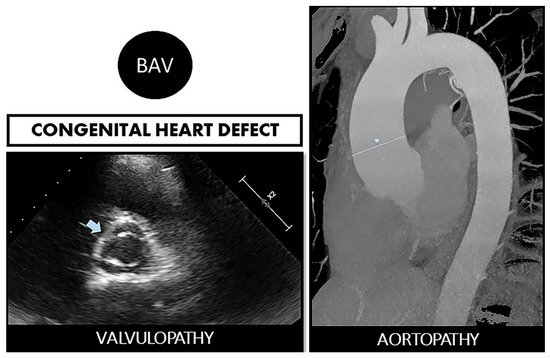
2]. With many cases in young people, it generates a high annual morbidity and mortality, derived not only from valve dysfunction (stenosis due to early calcification or aortic regurgitation) but also from aortic complications (). It has been shown, depending on the reports, that up to 35–80% of patients with BAV associate dilation of the ascending aorta [
3,
4]. Therefore, the patients have a higher age-adjusted relative risk of aortic dissection than the general population. Given the close relationship between valve alteration and its aortic continuity, the term “bicuspid aortopathy” has begun to be used in the literature [
5].
Figure 1. Bicuspid aortopathy is a double road. The congenital defect can develop in the person who suffers from both valvulopathy (stenosis or regurgitation) and aortopathy (aneurysms and risk of spontaneous dissection). On the left, echocardiographic image of BAV (arrow). On the right, a resonance image of dilated ascending aorta (marked straight line).
Classically, an autosomal dominant inheritance has been described, but appears with reduced penetrance (same genetic variant but no disease) and variable expressivity (same genetic variant with different manifestations of disease) [
6]. In any case, it is recognized that screening in family of the index case is necessary because the prevalence of BAV in first-degree relatives is 10 times higher than that in the general population [
7].
Since only symptomatic patients seek medical attention, there is a knowledge gap about the real consequences of having BAV, and thus, many patients remain underdiagnosed. BAV is generally diagnosed in adulthood with the onset of aortic valvular dysfunction [
8]. Practicable methods include both transthoracic (TTE) and transesophageal echocardiography (TEE), cardiac computed tomography (CT), and cardiac magnetic resonance (CMR).
More than 50% of patients with BAV undergo aortic valve replacement during their lifetime, and more than 25% of patients with BAV undergo aortic surgery performed for dilation of the aortic root or ascending aorta, often concurrent with aortic valve replacement [
4,
9].
In the last decade, many studies have been published focusing on the finding genetic and molecular alterations present in families with BAV and consistent inheritance, through registries of patients with BAV and tricuspid aortic valve (TAV), looking for differences between them [
10]. Isolated mutations, several genes, cellular pathways, different microRNAs, histopathological alterations, etc. have been found associated with the bicuspid aortopathy. Many pieces of this immense puzzle are unraveled in the following pages, being aware that in the coming years, the task will consist of interconnecting these elements. Consequently, we can say that despite intense research in the recent years, the manifest genetic, epigenetic, and molecular heterogeneity and complexity of this pathology means that the final pathogenic and ontogenetic mechanisms are still not fully understood. This article reviews the latest and outstanding advances in molecular and genetic investigations on bicuspid aortopathy.
2. BAV and Aortopathy: A Two-Way Road
The etiology and pathogenesis that lead to dilation of the ascending aorta in patients with a BAV are uncertain to date. The actual incidence and prevalence of aortopathy are not truly known either, since it is often an alteration with an asymptomatic course. Although, as classic studies explain, aortic dilation is a relatively common complication in patients with BAV, appearing at an earlier age and progressing continuously and rather faster [
11,
12]. Two theories have been proposed, two non-exclusive roads.
One of the roads is the “genetic theory”. As we have explained later, this theory defends the existence of mutations in different genes in the patients who carry a double leaflet valve and that affect the aorta at the same time. This connection should not be surprising since the aortic valve and the ascending aorta share the same embryological origin [
13].
The other road is the “hemodynamic theory”. Due to the anomalous opening of the BAV, an eccentric flow is formed directed against the aortic wall, this constant tangential force being the one that ends up generating the dilation. The consequent load is known as “wall shear stress” (WSS) [
14].
In the past decade, using 4D flow cardiovascular magnetic resonance (CMR 4D), it was found that the flow patterns in the ascending aorta of the patients with RL-BAV (fusion subtype of the right and left coronary cusps) and RN-BAV (fusion subtype of the right and non-coronary cusps) were different: RL-BAV impacted the anterior aortic wall and RN-BAV influenced directly on its posterior side, explaining different aortic dilation morphotypes [
15,
16]. However, there are also studies reporting a weak or no association between the BAV morphotype and the location of the aneurysm [
17,
18]. To elucidate these knowledge gaps, many subsequent studies continue to emerge in the recent years in this field. However, the subtypes according to the location of the raphe affect aortic flow. Moreover, valve degeneration has been shown to affect flow and WSS, being more severe and rotational in the patients with severe stenosis independently of valve morphology [
19].
It has been suggested in BAV that aortic dilation development is an adaptive mechanism of the arterial wall. A remodeling through mechano-sensing is possible. Regions with increased WSS have an extracellular matrix dysregulation and elastic fiber degeneration, with higher levels of MMP-2, MMP-3, and TGF-β in the areas with the higher WSS, associated with a greater loss of architecture in the middle layer [
20,
21]. It has also been observed that patients with BAV-aortic stenosis present increased WSS and thinning and loss of elastic fiber architecture more markedly than those with BAV-aortic regurgitation. In addition, the regional histopathology alteration manifests itself more strongly in mildly aortic dilation (<4.5 cm) [
22,
23].
However, not all patients with the same valvular phenotype have the same pattern of aortopathy and, furthermore, 25–35% of BAV present a non-dilated aorta in the follow-up [
3,
24]. However, controversies continue to exist. For example, first-degree relatives (with TAV) of patients with BAV suffer more frequently from aortic aneurysms, without presenting these eccentric flows involving WSS [
25]. In a recent meta-analysis by Girdauskas et al., the appearance of aortic dissection on post-aortic valve replacement was evaluated in the patients with BAV. It was found that those with regurgitation had a higher risk of dissection at follow-up, which could indicate greater tissue weakness. This occurs, even with the new valve replaced, where the WSS secondary to the altered outflow is not present. In contrast, BAV stenosis-associated aortopathy seems to follow a more benign course post-aortic valve replacement [
26].
Other non-hemodynamics factors must be linked. Currently, the results do not clearly indicate that hemodynamic effects on the aorta, due to outflow eccentricity, explain bicuspid aneurysms by themselves. Longer follow-up records (even decades) of patients with BAV are necessary to achieve clarity about the relationship of WSS and aneurysms, especially in non-dysfunctional BAVs.
It seems more sensible to think that the two phenomena (genetics + hemodynamics) participate. The impaired aortic ejection flow, with a weak arterial wall that is already genetically determined or histo-molecularly altered, will possibly cause or accelerate the development of aneurysms (). Understanding the mechanisms underlying vascular remodeling of the aortic wall is essential for the development of new risk scales, not based on measurements of aortic size, but on imaging parameters or biomarkers, which allow establishing the best individualized surgical time.
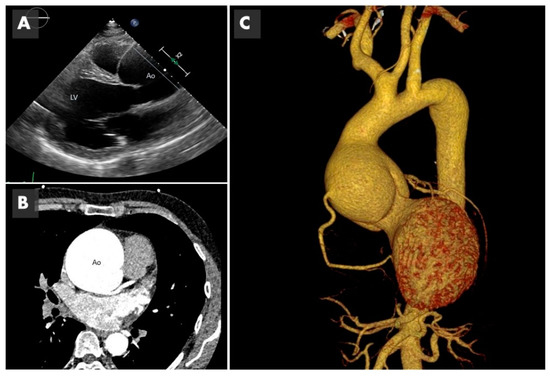
Figure 2. Imaging of bicuspid associated aneurysm. (A) The 2D image of a transthoracic echocardiogram in the parasternal long axis plane showing the dilated aorta (Ao) marked with the straight line (LV: left ventricle). (B) Computed tomography (CT) in axial plane showing the very dilated ascending aorta (Ao) at the level of the sinuses of Valsalva. (C) The 3D CT reconstruction, showing a huge 8 cm aneurysm.
3. Future Directions
The search for the ultimate genetic or epigenetic cause of the different bicuspid phenotypes should be facilitated by the next-generation sequencing tools that allow study of large populations at low cost.
In the near future, studies should also be directed to improve diagnostic and stratification criteria to better predict a more precise personalized risk for BAV patients. To achieve this proposal, imaging methods around the use of modern techniques, such as CMR-4D, are essential [
107].
The novel field of the ncRNAs requires further studies to fully understand how they are regulated and the molecular mechanisms through which they carry out their action. Uncovering the significance of ncRNAs in the origin of the BAV aortopathy would not only allow a path to find biomarkers for earlier diagnosis, prior to the appearance of symptoms, but also contribute to the development of new RNA-based disease therapies [
68].
Additional omic studies in plasma will be essential for the identification of more precise biomarkers that could be used in clinical practice. In addition, basic research with cell and/or animal models should continue in the direction of clarifying the molecular pathways that lead to valve degeneration in order to identify therapeutic targets that are absolutely lacking.