Epidemiological literature suggests a protective effect of omega-3 (n-3) polyunsaturated fatty acids (PUFAs) against cancer. They are attributed to have significant anti-inflammatory properties, and are reported to directly inhibit carcinogenesis and tumor expansion, whilst also reducing the risk for secondary complications, thus representing a promising approach for adjunctive chemotherapy treatment. At the same time, the incidence of malnutrition amongst children with cancer is high and both under- and overnutrition are associated with detrimental consequences, including increased risks for morbidity and mortality, early relapse rates, and a higher prevalence of secondary complications during treatment. Taken together with the benefits of n-3 PUFA supplementation, an enhancement of the nutritional status is a potentially modifiable prognostic factor in pediatric oncology.
- children
- nutrition
- omega-3 fatty acids
- oncology
1. Definition and Prevalence of Malnutrition in Childhood Cancer
2. Etiology, Pathophysiology, and Considerable Aspects of Nutrition in Children with Cancer
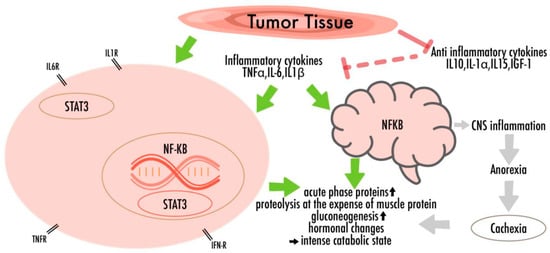
3. General Structural Features and Health Advantages of n-3 PUFAs
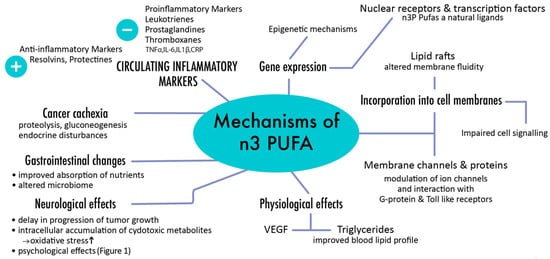
4. Role of PUFAs in Pediatric Oncology
5. Role of PUFAs during Chemotherapy
6. N-3 PUFAs in Primary and Secondary Prevention
7. Dosing of N-3 PUFA Supplementation
8. Potential Adverse Effects of n-3 PUFAs
References
- Vaughan VC, Hassing MR, Lewandowski PA Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer. 2013;108(3):486-492. doi:10.1038/bjc.2012.586
- Schoeman J. Nutritional assessment and intervention in a pediatric oncology unit. Indian J Cancer. 2015;52(2):186-190. doi:10.4103/0019-509X.175832
- Rogers PC, Barr RD. The relevance of nutrition to pediatric oncology: A cancer control perspective. Pediatr Blood Cancer. 2020;67(S3). doi:10.1002/pbc.28213
- Triarico S, Rinninella E, Cintoni M CMA. Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci. 2019;(23):1165-1175.
- Lenat Joffe EJL. Nutrition during childhood cancer treatment: current understanding and a path for future research. Lancet Child Adolesc Heal. 2020;4(6):465-475.
- Gaynor EPT, Sullivan PB. Nutritional status and nutritional management in children with cancer. Arch Dis Child. 2015;100(12):1169-1172. doi:10.1136/archdischild-2014-306941
- Selwood K, Ward E, Gibson F Assessment and management of nutritional challenges in children’s cancer care: A survey of current practice in the United Kingdom. Eur J Oncol Nurs. 2010;14(5):439-446. doi:10.1016/j.ejon.2010.04.004
- Morland SL, Martins KJB, Mazurak VC. N-3 Polyunsaturated Fatty Acid Supplementation During Cancer Chemotherapy. J Nutr Intermed Metab. 2016;5:107-116. doi:10.1016/j.jnim.2016.05.001
- Galati PC, Resende CMM, Salomão RG, Scridelli CA, Tone LG, Monteiro JP. Accurate determination of energy needs in children and adolescents with cancer. Nutr Cancer. 2011;63(2):306-313. doi:10.1080/01635581.2011.523505
- Gorjao R, dos Santos CMM, Serdan TDA , et al. New insights on the regulation of cancer cachexia by N-3 polyunsaturated fatty acids. Pharmacol Ther. 2019;196:117-134. doi:10.1016/j.pharmthera.2018.12.001
- Perdomo CAS. Sociodemographic and clinical characteristics associated with vitamin D status in newly diagnosed pediatric cancer patients. Pediatr Hematol Oncol. 2020;37(4):314-325. doi:10.1080/08880018.2020.1721629
- Ward Evelyn HLM. Nutritional support in children and young people with cancer undergoing chemotherapy. Cochrane Database. 2015;(8).
- Edward P T Gaynor PBS. Nutritional status and nutritional management in children with cancer. BMJ. 2015;(100):1169-1172.
- Burgerhof TBSIj. Eating and feeding problems in children with cancer: Prevalence, related factors, and consequences. Clin Nutr. 2020;39(10):3072-3079. doi:10.1016/j.clnu.2020.01.012
- J. Scarlett DLMG. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100(5):478-489. doi:10.1016/j.physbeh.2010.03.011
- Barr RD, Stevens MCG. The influence of nutrition on clinical outcomes in children with cancer. Pediatr Blood Cancer. 2020;67(S3):1-11. doi:10.1002/pbc.28117
- Farahat E-HEI. ω-3 fatty acids as an adjuvant therapy ameliorates methotrexate-induced hepatotoxicity in children and adolescents with acute lymphoblastic leukemia: A randomized placebo-controlled study. Nutrition. 2016;32(1):41-47. doi:10.1016/j.nut.2015.06.010
- Barr RD, Gomez-Almaguer D, Jaime-Perez JC, Ruiz-Argüelles GJ. Importance of Nutrition in the Treatment of Leukemia in Children and Adolescents. Arch Med Res. 2016;47(8):585-592. doi:10.1016/j.arcmed.2016.11.013
- Gu Z, Shan K, Chen H, Chen YQ. n-3 Polyunsaturated Fatty Acids and Their Role in Cancer Chemoprevention. Curr Pharmacol Reports. 2015;1(5):283-294. doi:10.1007/s40495-015-0043-9
- Nabavi SF, Bilotto S, Russo GL, et al. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34(3):359-380. doi:10.1007/s10555-015-9572-2
- Raquel RI, Laura3 W, David W. Citation: A systematic review of N-3 and N-6 Polyunsaturated saturated Fatty Acid Concentration in Childhood Cancer Patients and Associated Clinical Outcomes. EC Nutr. 2019;14.9:709-722. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016035210;
- Kolacz CM. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int J Food Sci Nutr. 2015;66(6):611-622. doi:10.3109/09637486.2015.1077790
- Sterescu, Rousseau-Harsany, Farrell, Powell, David D. The potential efficacy of n-3 fatty acids as anti-angiogenic agents in benign vascular tumors of infancy. Med Hypotheses. 2006; 66: 1121-1124.
- Vaculova HS. Docosahexaenoic fatty acid (DHA) in the regulation of colon cell growth and cell death: A review. Biomed Pap. 2012;156(SUPPL. 3):186-199. doi:10.5507/bp.2012.093
- Deckelbaum RJ, Worgall TS, Seo T. N-3 Fatty Acids and Gene Expression. Am J Clin Nutr. 2006;83(6):1520-1525. doi:10.1093/ajcn/83.6.1520s
- Wang J, Luo T, Li S, Zhao J. The powerful applications of polyunsaturated fatty acids in improving the therapeutic efficacy of anticancer drugs. Expert Opin Drug Deliv. 2012;9(1):1-7. doi:10.1517/17425247.2011.618183
- Raquel D.S. Freitas MMC. Protective Effects of Omega-3 Fatty Acids in Cancer-Related complications. J Nutr. 2019;11(945).
- Oliviera V., Marinho R., Vitorino D., Santos G.A., Moraes J.C. DN. Diets containing alpha-linolenic (n-3) or oleic (n-9) fatty acids reduces obese mice from insuline resistance. Endocrinology. 2015; 156: 4033-4046.
- Nakamoto K., Nishinaka T., Ambo A., Mankura M., Kasuya F. TS. Possible involvement of beta-endorphin in docosahexaenoic acid-induced antinociception. Eur J Pharmacol. 2011; 666: 100-104.
- Raquel RI, Laura3 W, David W. Citation: A systematic review of N-3 and N-6 Polyunsaturated saturated Fatty Acid Concentration in Childhood Cancer Patients and Associated Clinical Outcomes. EC Nutr. 2019;14.9(August):709-722. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016035210;
- Ibrahim Bayram, Fatih Erbey, Nilgun Celik, Jeffrey L Nelson AT. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy-related weight loss in children. Pediatr Blood Cancer. 2009;52(5):571-574.
- Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269(2):363-377. doi:10.1016/j.canlet.2008.03.044
- Gleissman H. omega-3 fatty acid supplementation delays the progression of neuroblastoma in vivo. Int J Cancer2. 2010;128:1703-1711.
- Yang MG. Docosahexaenoic acid metabolome in neural tumors: identification of cytotoxic intermediates. FASEB J. 2010;24(3):906-915. doi:10.1096/fj.09-137919
- Chen X., Wu S., Chen C., Xie B., Fang Z. HW. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting HMGB1/TLR4/NFkB pathway following experimental traumatic brain injury. J Neuroinflamm. 2017;14(143).
- Kobayakawa M., Yamawaki S., Hamazaki K., Akechi T., Inagaki M. UY. Levels of Omega-3 fatty acid in serum phospholipids and depression in patients with lung cancer. Br J Cancer2. 5AD;93:1329-1333.
- Müller C.P., Reichel M., Mühle C., Rhein C., Gulbins E. KJ. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851:224-228.
- Bigornia S.J., Harris W.S., Falc L.C. TKL. The Omega-3 index is inversly associated with depressive symptoms among individuals with elevated oxidative stress biomarkers. J Nutr. 2016;146:758-766.
- Zhang Y, Zhang B, Dong L, Chang P. Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis. Adv Nutr. 2019;10(1):133-147. doi:10.1093/advances/nmy076
- Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fat Acids. 2008;79(3-5):101-108. doi:10.1016/j.plefa.2008.09.016
- Calder PC, Yaqoob P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors. 2009;35(3):266-272. doi:10.1002/biof.42
- Saray Gutierrez SLS und MEJ. Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci. 2019;20(5028).
- Segerström HG. Omega-3 fatty acid supplementation delays the progression of neuroblastoma in vivo. Int J Cancer. 2011;128(7):1703-1711. doi:10.1002/ijc.25473
- Brinksma A, Sanderman R, Roodbol PF, et al. Malnutrition is associated with worse health-related quality of life in children with cancer. Support Care Cancer. 2015;23(10):3043-3052. doi:10.1007/s00520-015-2674-0
- Salvador C, Entenmann A, Salvador R, Niederwanger A, Crazzolara R, Kropshofer G. Combination therapy of omega-3 fatty acids and acipimox for children with hypertriglyceridemia and acute lymphoblastic leukemia. J Clin Lipidol. 2018;12(5):1260-1266. doi:10.1016/j.jacl.2018.05.021
- Bauer J., Jürgens H., Frühwald M.C. Important aspects of Nutrition in children with cancer. Nutrition 2011 Mar; 2(2): 67–77.
- Lange B. et.al Mortality in Overweight and Underweight Children With Acute Myeloid Leukemia JAMA Journal of the American Medical Association 293(2):203-11
- Yelena P. Wu, James P. Franciosi, Marc E. Rothenberg Behavioral feeding problems and parenting stress in eosinophilic gastrointestinal disorders in children Pediatric Allergy and Immunology August 2012: 23(8)
- Horvath A. Chemotherapy induced liver toxicity in children with malignant diseases Bulletin of Medical Sciences June 2018: 91(1)
- Lavriv D.S. et.al Should omega-3 fatty acids be used for adjuvant treatment of cancer cachexia? Clinical Nutrition ESPEN March 2018:15
- Raquel D.S. Freitas, Maria M. Campos Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications Journal of Nutrients April 2019: 11(5) 945
- Segatto M. et.al Epigenetic targeting of bromodomain protein BRD4 counteracts cancer cachexia and prolongs survival Nature Communications November 2017: 8 (1707)
- White James P. et.al Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK Endocrinology and Metabolism May 2013
- Fappi A. et.al Omega‐3 multiple effects increasing glucocorticoid‐induced muscle atrophy: autophagic, AMPK and UPS mechanisms Physiological reports January 2019
- Whitehouse A. et.al Downregulation of Ubiquitin-Dependent Proteolysis by Eicosapentaenoic Acid in Acute Starvation Biochemical and Biophysical Research Communications July 2001 785 (3)
- Tisdale et.al Attenuation of muscle atrophy in a murine model of cachexia by inhibition of the dsRNA-dependent protein kinase British Journal of Cancer April 2007 96 (8): 1216-22
- Tisdale M.J. Catabolic mediators of cancer cachexia Current Opin Support Palliat Care December 2008 2 (4) 256-61
- Horvath A. et.al ω-3 Fatty Acid Supplementation Does Not Affect Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis The Journal of Nutrition March 2017 147 (3) 167-176
- Chmielewska A. et.al Effects of prenatal and/or postnatal supplementation with iron, PUFA of folic acid on neurodevelopment: update British Journal of Nutrition January 2016 122
- Bloch M.H. et.al Omega-3 Fatty Acid supplementation for the Treatment of Children with Attention-Deficit/Hyperactivity Disorder Symptomatology: Systematic Review and Meta-Analysis J Am Acad Child Adolesc Psychiatry October 2011 50 (10) 91-1000
- Bloch M.H. et.al Nutritional Supplements for the Treatment of Attention-Deficit Hyperactivity Disorder Child Adolesc Psychiatr Clin N Am October 2014 23 (4) 83-897
- Rogers L.K., Valentine C.J. Keim S.A. DHA supplementation: Current implications in pregnancy and childhood Pharmacological Research April 2013 70 (1) 13-19
- Ling Tao Oxidation of Polyunsaturated Fatty Acids and its Impact on Food Quality and Human Health Adv Food Technol Nutr Sci Open J. 2015 1(6)135-142
- Lange K., Nakamura Y. Are there serious adverse effects of omega-3 polyunsaturated fatty acid-supplements? Journal of Food Bioactives September 2019 7 1-6
- Wachira John K., Larson Mark K., Harris William S. N-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights Cambridge University Press January 2014 111 (9)
- Van Dijk S. et.al Effect of prenatal DHA supplementation on the infant epigenome: results from a randomized controlled trial Clin Epigenetics November 2016 4 (8) 114
- Robinson SL et.al Maternal fatty acid concentrations and newborn DNA methylation Am J Clin Nutr. March 2020 111 (3) 613-621
- Katanic Stankovic J.S. et.al Antioxidant Supplementation in the Treatment of Neurotoxicity Induced by Platinum-Based Chemotherapeutics – A Review Integr Cancer Ther March 2016 15(1) 17-39
- Yasueda, Urushima, Toshinoro Efficacy and Interaction of Antioxidant Supplements as Adjuvant Therapy in Cancer Treatment Oxid Med Cell Longev. 2016 6719534
- Mut-Salud et.al Antioxidant Intake and Antitumor Therapy: Toward Nutritional Recommendations for Optimal Results Oxid Med Cell Longev. November 2022 DOI: 10.1155/2016/6719534
- D’Angelo S., Mottia M.L., Meccariello R. n-3 and n-6 Polyunsaturated Fatty Acids, Obesity and Cancer Nutrients August 2020 12 2751
- Barnes M. et.al Inhibition of neuroblastoma cell proliferation with omega-3 fatty acids and treatment of a murine model of human neuroblastoma using a diet enriched with omega-3 fatty acids in combination with sunitinib Pediatric Research January 2012 71 198-178
- Simopoulos AP The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. October 2002 56(8) 365-379
- Iniesta R.R. et.al Effects of pediatric cancer and its treatment on nutritional status: a systematic review Nutrition reviews 2015 73 (5) 76-295
- Brinksma A. et.al Malnutrition is associated with worse health-related quality of life in children with cancer Supportive Care in Cancer 2015 23 3043-3052
- Robison LL, Bhatia S. Late-effects among survivors of leukaemia and lymphoma during childhood and adolescence. Br J Haematol. 2003;122:345–359.
- Revuelta,Iniesta, et.al Nutritional status of children and adolescents with cancer in Scotland: A prospective cohort study March. 2015 DOI: 10.1093/nutrit/nuu062.
- Brinskma et.al Changes in Nutritional status in childhood cancer patients: a prospective cohort study 2015 DOI: 10.1016/j.clnu.2014.01.013
- Iniesta RR et.al Micronutrient status influences clinical outcomes of pediatric cancer patients during treatment: A prospective cohort study 2021 DOI: 10/1016/j.clnu.2021.03.020
- Barnea et.al Obesity and Metabolic Disease After Childhood Cancer Oncology November 2015 29(11): 849–855.
This entry is adapted from the peer-reviewed paper 10.3390/nu13061800
