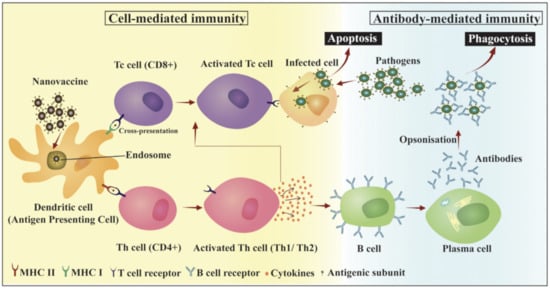
2.1. Polymer-Based Nanovaccines
Polymers have been extensively studied as components and excipients for vaccine platforms in the immunotherapy of various infectious diseases, immunotherapy, and cancer. Polylactide-co-glycolide (PLGA) polymer-based nanosystems are one example—the most famous—with numerous literature references. PLGA is a biocompatible and biodegradable polymer material because it is metabolized in the human body by enzymes in monomers of the lactic acid and the glycolic acid. PLGA polymer can self-assemble into different morphologies at nano- or micro-scale, which are strongly dependent on the preparation method, the aqueous medium, and the other components of the formulation [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66]. Namely, the PLGA formulations that are used as vaccine (and/or drug) delivery platforms are (functionalized) nanoparticles, nanospheres, nanoemulsions, micelles, and (nano/hydro)gels. [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66]. The physicochemical characteristics, the solubility, and the thermodynamic/physicochemical stability of PLGA nanosystems can be fine-tuned extensively. Further, PLGA can be conjugated with polyethylene glycol (PEG) or polyetherimide to form block copolymers, which can self-assemble into polymeric micelles, and the resulting micellar nanoparticles can incorporate hydrophobic molecules and hydrophobic peptide antigens or proteins [
11,
12,
13,
14]. summarizes the different types of nanoparticles, their characteristics, and their disadvantages. We present and analyze different examples from the recent literature.
Table 1. Characteristics of different types of nanoparticles used in vaccines. (Adapted from [
65]).
|
Types
|
Size
|
Shape
|
Bio-Toxicity
|
Biocompatibility
|
Disadvantages
|
|
VPLs
|
20–800 nm
|
Particle
|
Some unpredictable
consequences
|
Highly cross-protective antibody responses
|
Low immunogenicity
|
|
SAP
|
10 nm length (70 nm) and wide (40 nm)
|
Spherical or barrel
|
-
|
Reduce the risk of immunogenicity; improve half-life
|
Creates a new molecular entity; poor solution stability and aggregation
|
|
CNPs
|
30–60 nm length (100–1000 nm) and diameter (0.8–2.0 nm)
|
Fullerene particle, Nanotube, or mesoporous spheres
|
Negligible effect on cell viability
|
Good biocompatibility
|
Low immune function
|
|
GNs
|
2–150 nm
|
Particle, rod, spherical, and cubic
|
Immuno-toxicity
|
More potent immune response
|
Immuno-toxicity
|
|
CNs
|
50–100 nm
|
Calcium phosphate particle
|
Biocompatible and safe
|
Biocompatibility and easily biodegradable
|
-
|
|
SNs
|
50–20 nm
|
Tunable hollow and mesoporous structure
|
Toxicity degraded
|
Biocompatibility and selective tumor targeting, real-time multimodal imaging, vaccine delivery
|
Toxicity derived from the reducing agents
|
|
LSs
|
25–1000 nm
|
Spherical
|
Safely degraded
|
Stabilize the antigen, biocompatible and stable
|
-
|
|
Polymer
|
10–2000 nm
|
Particles
|
Non-toxicity
|
Antigen loading into polymeric particles under aqueous conditions via a self-healing process
|
Loss of antigenicity and immunogenicity during particle synthesis
|
|
ISCOM
|
40 nm
|
Cage-like particles
|
Cytotoxicity-mediated immune responses
|
-
|
Cytotoxicity-mediated immune responses
|
|
EN
|
50–600 nm
|
Cytotoxicity-mediated immune responses
|
Safe
|
Safe and potent
vaccine adjuvant
|
-
|
VLPs, virus-like particles; SAP, self-assembled protein; CNPs, carbon nanoparticles; GNs, gold nanomaterials; CNs, calcium nanoparticles; SNs, silica nanoparticles; ISCOM, immunostimulating complex; LSs, liposomes; EN, emulsion.
Porous poly(lactic-co-glycolic acid) (PLGA) and poly(L-lactic acid) (PLA) nanoparticles have been investigated for pulmonary delivery of hepatitis B vaccine [
15]. Three different formulations of PLA and PLGA nanoparticles containing a standard amount of hepatitis B surface antigen (HBsAg) were designed, developed, and prepared by a double-emulsion-solvent-evaporation method. The immune responses were studied by quantitating the secretion of IgA in fluids of mucosa and measuring cytokine levels in mice spleen homogenates. The nanoparticle hydrophilicity/hydrophobicity on mucosal and cell-mediated immune responses was also investigated. Namely, the hydrophobic nanoparticles with a size larger than 500 nm elicited a more robust increase in IgA, interleukin-2, and interferon-γ levels compared to hydrophilic nanoparticles with a size smaller than 500 nm. According to the described results the prepared inhalable polymeric nanoparticles of HBsAg exhibit an enhancement of immune responses [
15]. In other words, the prepared aerosolized and inhaled PLA and PLGA nanoparticles enhance the responses (humoral, mucosal, and cytokine) to hepatitis B vaccine [
15].
Diwan et al. investigated the co-delivery of CpG synthetic oligodeoxynucleotides and antigens in biodegradable nanospheres as an alternative approach for immunization, using tetanus toxoid as the model antigen and oligodeoxynucleotide (ODN) #1826 as the model CpG sequence. The results suggested that the co-delivery of CpG ODN adjuvants and antigens in nanospheres is a more efficient delivery approach for immunization than the use of the antigens alone in dispersion state [
16]. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate-coated chitosan nanoparticles was also achieved by Borges et al. [
19]. Alginate-coated chitosan nanoparticles were loaded with the recombinant hepatitis B surface antigen (HBsAg) and applied to mice by the intranasal route. All intranasally vaccinated groups showed higher interferon-γ secretion when compared to naive mice [
16].
Poly(lactide-co-glycolide) (PLGA) nanoparticles were used for the delivery of a stable immunogenic domain 4 of protective antigen (PAD4) of Bacillus in order to overcome the issues of dosage, nanotoxicity of adjuvant, and the limited stability associated with anthrax vaccines according to a recent publication [
17]. The nanoformulations were prepared by water/oil/water solvent evaporation method. The PAD4 systems induced an IgG response with mixed IgG1 and IgG2a subtypes, whereas the control PAD4-immunized mice elicited low IgG response with predominant IgG1 subtype. The PAD4 systems also induced both Th1 and Th2 responses, whereas PAD4 elicited predominantly Th2 response [
17]. The effectiveness and the efficacy of this single-dose vaccine nanoformulation were compared with those of the recombinant PAD4 in providing protective immune response against a lethal challenge with Bacillus anthracis spores; the median survival of PAD4-NP-immunized mice was 6 days as compared to 1 day for PAD4-immunized mice [
17].
According to Lima et al., mice treated with viable Mycobacterium tuberculosis with no glycolipid trehalose dimycolate (TDM) on the outer cell wall (delipidated Mycobacterium tuberculosis) by intraperitoneal and intratracheal inoculation presented intense recruitment of polymorphonuclear cells into the peritoneal cavity and acute inflammatory reaction in the lungs, respectively [
18]. TDM-loaded biodegradable PLGA microspherical particles as well as TDM-coated charcoal particles induced an inflammatory reaction. Microspheres were prepared using the emulsion solvent evaporation technique. In addition, high levels of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-alpha), IL-12, IL-10, interferon-γ, and IL-4 production were detected in lung cells, and nitric oxide (NO) production was high in culture supernatants of bronchoalveolar lavage cells [
18,
19].
Alginate–poly(ethylenimine) (PEI) is a bio-reducible polymer material self-assembled into nanogel formulation for antigen loading and delivery vehicle that significantly improves vaccine-elicited humoral and cellular immune responses [
20]. The alginate–poly(ethylenimine) nanogels were formulated by the well-known technique of the electrostatic interaction of negatively charged sodium alginate with branched bioreducible cationic PEI followed by disulfide cross-linking to formulate bioreducible nanogels [
20]. This nanoplatform ameliorates vaccine-induced antibody secretion and CD8
+ T-cell-mediated tumor cell lysis. For this reason, this polymer vehicle could serve as a potent adjuvant system to improve vaccine-elicited humoral and cellular immune responses [
20].
Hasegawa et al. developed a complex system composed of cholesterol-bearing hydrophobized pullulan and the protein NY-ESO-1 [
21]. This protein belongs to a class of cancer/testis antigens and has been investigated as an immunogenic molecule in patients with different cancer types. From the in vitro experiments, the stimulation of CD8 and CD4 T cells from peripheral blood mononuclear cells in healthy volunteers with autologous of cholesterol-bearing hydrophobized pullulan/ESO-loaded dendritic cells as APCs were also investigated. The results were very promising for the development of a polyvalent cancer vaccine [
21].
Saad et al. showed the ability of Advax adjuvant, a novel polysaccharide adjuvant based on delta inulin, to enhance the immunogenicity of hepatitis B surface antigens (HBs) in mice and guinea pigs in comparison to the traditional alum adjuvant [
22]. Enhanced immune response and protective effects of nanochitosan-based DNA vaccine encoding T cell epitopes of Esat-6 and FL against Mycobacterium tuberculosis infection have also appeared in the literature [
23]. The immunized mice remarkably elicited enhanced T-cell responses and protection against Mycobacterium tuberculosis challenge [
23]. A hyperbranched polyglycerol multifunctionalized by “click chemistry” was synthesized, and a tumor-associated MUC1 glycopeptide combined with the immunostimulant T-cell epitope P2 from tetanus toxoid was loaded [
24]. This globular polymeric system exhibited a flexible dendrimer-like morphology, which allowed optimal antigen presentation to the immune system and strong immune responses in mice and IgG antibodies recognizing human breast-cancer cells [
24].
Poly(ethylene glycol)-b-poly(L-lysine)-b-poly(L-leucine) (PEG-PLL-PLLeu) polypeptides were self-assembled into micelles with significant cationic surface charge. The aforementioned hybrid polypeptides were designed as vaccine delivery platforms [
25]. The authors proved that the prepared polypeptide cationic micellar formulations robustly enhanced vaccine-induced antibody secretion by 70–90-fold, which could be due to their capability of inducing different biological pathways of the immune system (i.e., dendritic cell maturation, improving antigen uptake and presentation to APCs, promoting germinal center formation) [
25].
Zhang et al. formulated an “easy-to-adopt” strategy to enhance immune responses using functionalized alginate nanoparticles. The functionalized alginate nanoparticles were prepared by cross-linking of two different types of alginate using CaCl
2 [
26]. The mannose modified alginate was utilized for the specific targeting to the DCs. The authors also used ovalbumin (OVA) as model antigen and conjugated it to alginate molecules via the mechanism of pH-sensitive Schiff base bond. The above-described delivery platform was studied as a potential vaccine for cancer immunotherapy because it was found to increase the cross-presentation of OVA to B3Z T-cell hybridoma in vitro [
27]. The subcutaneous administration of this nanovaccine also induced a strong cytotoxic T-lymphocyte response and the parallel inhibition of E.G7 tumor growth in C57BL/6 mice [
26]. These pH-responsive alginate nanoparticles exhibit an added value to cancer immunotherapy due to spatiotemporal control of the incorporated antigen [
27]. According to Démoulis et al., alginate-coated chitosan nanogel has the ability to control the effect of toll-like receptor (TLR) ligands on blood dendritic cells [
28]. The findings of the experimental procedure showed that the influence of alginate-coated chitosan nanogels on human blood DC endocytosis of the TLR ligands was apparently a major contributory element. The last observation demonstrates the significance of predefining the interplay between delivery platforms and the immunostimulatory compounds for ensuring appropriate immune activation and efficacious combinations [
28]. The same group prepared alginate-coated chitosan nanogel formulations for the encapsulation of CpG-oligodeoxynucleotides (class-A or class-B CpG-ODNs). The results of the immune response of these platforms of nanogels were compared with the same free CpG-ODNs or with pure nanogels [
29]. Experiments were performed on both porcine and human blood DC subpopulations. Incorporation of class-A CpG-ODN into alginate nanogels significantly reduced the CpG-ODN uptake and intracellular trafficking in the cytosol. On the contrary, incorporation of class-B CpG-ODN increased its uptake and did not consistently influence intracellular trafficking into the nucleus. The selection of the CpG-ODN system as an adjuvant form is thus very important in terms of how it will behave with nanoparticulate vaccine delivery systems, exhibiting distinctive modulatory influences on the CpG-ODN [
29].
3.2. The Added Value of Polymer-Based Nanovaccines
We presented several examples from the recent literature about polymer-based nanovaccines and in some cases polymer microparticulate vaccine platforms. Generally, different types of nanoparticles have been already used as antigen vehicles and/or particulate adjuvant in the field of novel vaccines. As mentioned before, summarizes the different types of nanoparticles, their characteristics, and their disadvantages. Additionally, we presented several polymers with different architectures used as vaccine platforms in the previous section, but a high percentage of the published studies deal with PLGA. summarizes the main advantages and disadvantages of PLGA-based particulate vaccine delivery systems.
Table 2. Summary of the main advantages and disadvantages of PLGA-based particulate vaccine delivery systems. (Adapted from [
66]).
|
Advantages
|
Disadvantages
|
-
PLGA polymers are biodegradable, widely available, and approved by regulatory agencies such as the FDA
-
PLGA particles for delivery of several different agents are on the market
-
PLGA particles can be administered via various routes
-
PLGA particles may decrease toxicity of vaccine components
-
Particle size, surface, and/or release characteristics can be tailored
-
PLGA particles allow controlled antigen release
-
PLGA particles protect antigen from degradation and elimination
-
PLGA particles enhance antigen uptake by APCs by mimicking size and shape of pathogens
-
PLGA particles enhance and prolong antigen cross-presentation efficiency
-
PLGA particles allow concomitant delivery of multiple vaccine components
-
Large surface area and surface functional groups allow conjugating of targeting moieties
-
PLGA particles may lead to antigen dose sparing
|
-
Negative charge of PLGA particles is disadvantageous for particle uptake
-
PLGA particle preparation process must be tailored to the properties of the antigen
-
PLGA particles cannot be sterile filtered
-
Antigen degradation may occur during preparation, storage, and release
-
Antigen release is often incomplete
-
Particle aggregation may occur
-
Particle size may limit crossing of biological barriers
|
The polymer-based nanovaccines exhibit several advantages:
-
Strong cellular immune responses [
38,
42];
-
Increased secretion of cytokines [
58];
-
Different routes of administration [
47,
49,
50,
53];
-
Co-loading of antigens [
34,
52];
-
Prolonged antigen circulation [
43];
-
Increased levels of antibodies and antigen-specific antibodies (i.e., IgA, IgG, etc.) [
46,
47,
58];
-
Th1 and/or Th2 immune responses [
40,
51];
-
Advanced adjuvant properties [
38];
-
-
Other nanoparticles, e.g., liposomes, exhibit some of these advantages [
66,
67,
68,
69], but only polymer-based nanovaccines can present all the above advantages. Furthermore, comparative studies of biodegradable nanoparticles composed of poly(glutamic acid) nanoparticles with aluminum adjuvants, which are already used in commercial vaccines, showed an excellent antigen uptake by dendritic cells (localized in the lysosomal regions) and adjuvant activity, as well as induction of immune response in mice via a TLR4 and MyD88 signaling pathway [
70,
71]. These biodegradable nanoparticles are effective for carrying different types of antigens and also exhibit antigen-specific humoral and cellular immunity [
70,
71,
72,
73,
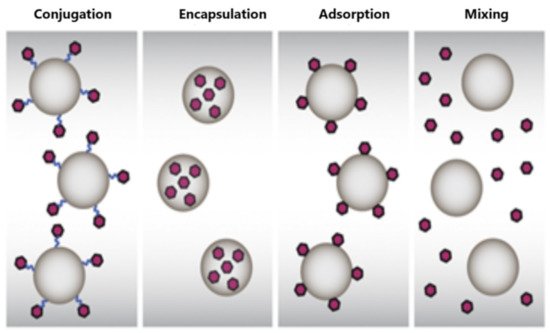
74]. The cellular and the humoral responses are strongly dependent on the architecture and the chemistry of the hydrophobic polymer chains and the formulation protocol of the nanoparticulate vaccine platform (encapsulation or mixture) [
75,
76]. The size of these nanoparticles plays a significant role in the uptake and activation behaviors of APCs migrating to lymph nodes and DC maturation [
77]. Namely, the sizes above 100 nm are ideal for cellular uptake, while the sizes below 100 nm are perfect for the maturation of DCs in lymph nodes [
77]. The surface coatings and/or surface decorations of nanogels influence the interferon-γ production by T lymphocytes [
78]. The presence of PEG as surface coating and the length of its chain influence the antibody–receptor interactions and induction of antigen-specific T-cell responses [
79]. Furthermore, the immunization with polymer nanoparticles induces high antibody rates compared to other nanosystems such as liposomes and alum [
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85].
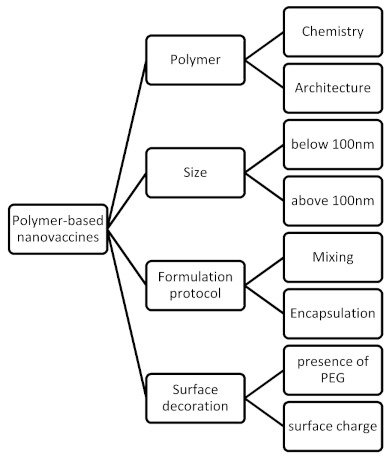
As mentioned above, from the technological point of view, polymers and, consequently, polymer-based nanoparticles offer design versatility. Firstly, there are different types (compositions and architectures) of polymers. Different types of nanoparticles can be prepared due to different types of polymers (i.e., micelles, polymersomes, hydrogels, polymeric nanoparticles, hybrid particles, etc.). The physicochemical characteristics of these nanosystems are crucial for their behavior in vitro and in vivo. The surface hydrophobicity and size are the most important formulation parameters for the creation of antigen-specific antibodies [
85]. The formulation parameters of the design and development of polymer-based nanovaccines are presented in . Additionally, the lower cost of polymers in comparison to other materials, i.e., lipids, dendrimers, etc., and their large-scale and commercial use make polymer-based nanovaccines attractive for the pharmaceutical companies from the development point of view.
Figure 2. The formulation parameters of the design and development of polymer-based nanovaccines.
3.3. The Limitations in the Development of Polymer-Based Nanovaccines
We have described several examples of polymer-based nanovaccines that have appeared in the literature in different stages of preclinical and clinical studies. The results and the outcomes were in most of the cited cases very optimistic for further development of polymer-based platforms for vaccine applications. On the other hand, the number of polymer-based nanovaccines is close to zero, while there are several polymer nanomedicines on the market [
84,
85,
86,
87]. Firstly, to push forward the application of polymer-based nanovaccines, it would be important that more groups from different fields (from synthetic chemists and formulation scientists to clinical doctors and regulatory scientists) be actively involved in the testing of new vaccine platforms against different infectious diseases. This collaboration is the first and important step to overcome the potential shortcomings of polymer-based nanovaccines. Other important issues deserving attention include the following: (a) The synthesis of new polymers with low nanotoxicity and low immunogenicity is one of the limitations of the usage of new polymers in the further development of polymer therapeutics [
84,
85,
86,
87]. (b) The in-depth investigation of the physicochemical and morphological properties of the prepared polymer formulations, by using specialized techniques, is mandatory for all the nanomedicines under development. This need increases the cost of the preclinical studies in comparison to other pharmaceutical formulations [
84,
85,
86,
87]. (c) The pharmaceutical industries should change or further develop the instrumentation for the production and quality control of the polymer-based nanovaccines. Scientists with qualification in the field of polymers are also required in big pharma at every stage of the design and the development of polymer-based nanovaccines [
84,
85,
86,
87]. Last but not least, the regulatory landscape is not very clear containing grey areas for the nanoformulations, and this is an additional difficulty for the preparation of the dossier of a new vaccine. All these limitations act as a brake towards the acceleration of the development of polymer-based nanovaccines [
84,
85,
86,
87,
88,
89,
90]. However, all these limitations could be a challenge and an opportunity for the strongest collaboration of scientists to overcome them.