Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Myocardial restoration approaches so far have encompassed various types of cells, cell products or derivatives, scaffolds of various physical conditions, as well as multiple administration routes.
- hydrogel
- extracellular matrix hydrogels
- myocardial infarctions
- myocardial infarction therapy
- cardiac stem cell therapy
- tissue engineering
- cell-based therapy
1. Introduction
The prevailing dogma suggesting that adult mammalian cardiomyocytes are post-mitotic cells with no ability to renew has been recently overthrown by studies demonstrating a low level of proliferation, even in adult hearts [1]. However, the regenerative capacity is minimal and insufficient to overcome the loss of cardiac cells following MI. The inability of the adult heart to regenerate has yielded several preclinical and clinical studies focused on different cell-based therapies. Despite very promising preclinical results, these results have so far not been translated into clinical practice. Some of the major challenges limiting their clinical application are low retention and survival rates; very limited trans-differentiation into cardiomyocytes; safety; and, in some cases, ethical concerns.
Over the last few decades, cell therapy has been applied in clinical myocardial restoration. Though the result is non-conclusive, some studies have shown the attenuation of ventricular remodeling. The ensuing hostile and inflammatory environment results in the rapid death of injected cells, or lack of integration thereof. It is incomprehensive, and the vast majority of studies have proven that injected cells do not organize in an integrated syncytium, which excites orchestrated contractility. Depending on the type of cells that are randomly injected, different complications occur [2]. Solid scaffolds—even when adding thickness to the aneurysmatic scar—have not proven themselves as a viable solution either, particularly due to the necessity of open heart surgery to implant them (Figure 1).
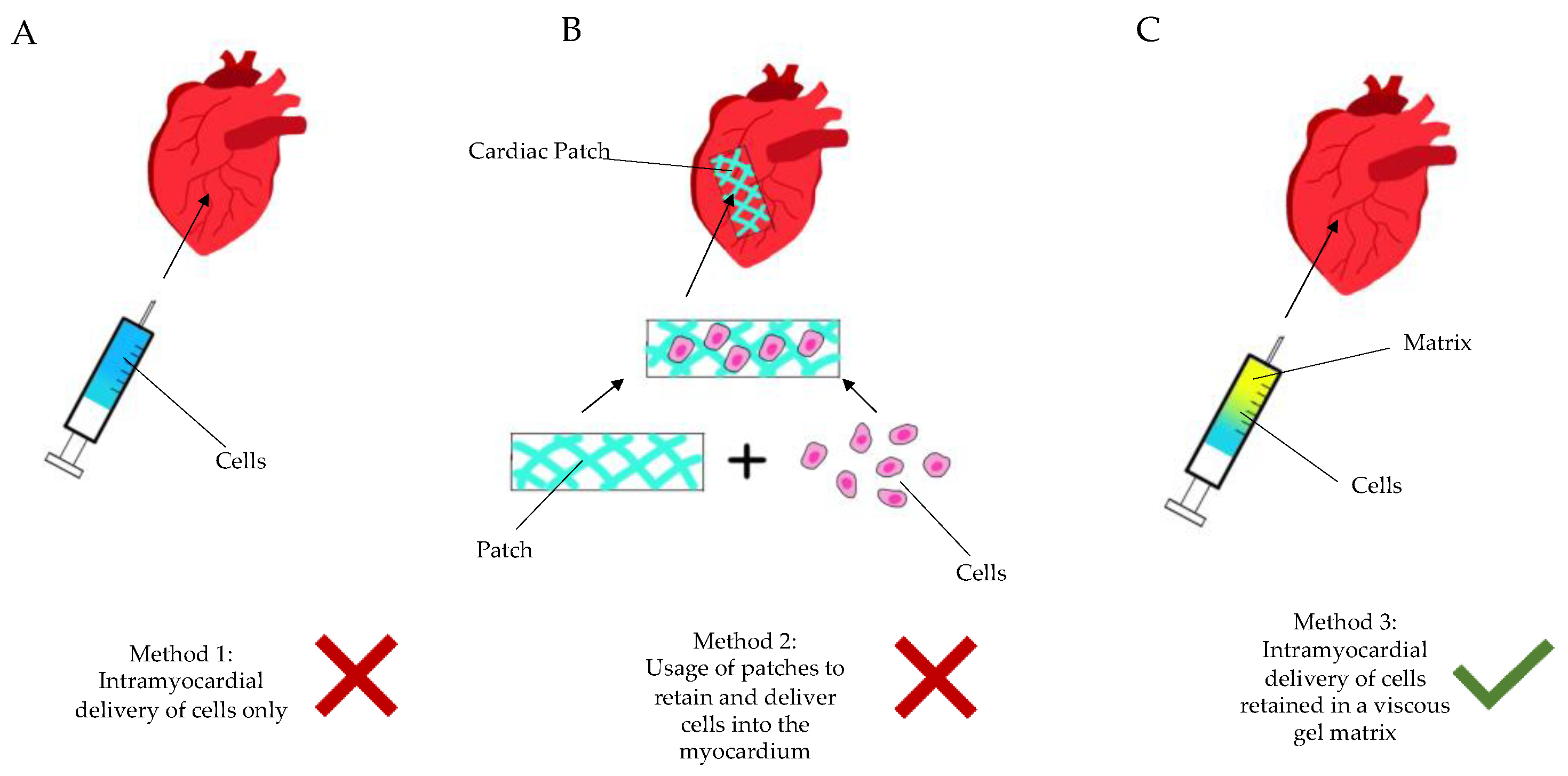 Figure 1. Diagram showing the current methods of regenerative cardiac therapy. (A): Method 1—intramyocardial delivery (injection) of stem cells only, without a retaining matrix. (B): Method 2—seeding of cells into a patch-like matrix, which is then implanted onto the epicardium via sutures or glue. (C): Method 3—intramyocardial injection of cells/active ingredient retained in a gel matrix, either during an open surgery (thoracotomy) or in a minimally invasive manner (percutaneously, etc.).
Figure 1. Diagram showing the current methods of regenerative cardiac therapy. (A): Method 1—intramyocardial delivery (injection) of stem cells only, without a retaining matrix. (B): Method 2—seeding of cells into a patch-like matrix, which is then implanted onto the epicardium via sutures or glue. (C): Method 3—intramyocardial injection of cells/active ingredient retained in a gel matrix, either during an open surgery (thoracotomy) or in a minimally invasive manner (percutaneously, etc.).There is an obvious need for a targeted, less invasive myocardial restoration treatment, which does not add too much stand-alone trauma to the patient and can be integrated into a viable clinical protocol, to be adopted by cardiologists as well. Arising from the above pain-points, we have long shifted our focus from stem cells to liquid compounds, with the following key value propositions:
-
Injectable, hence minimally invasive, administration
-
Autologous material, not of stem cell nature, to be derived simply during treatment
-
A polytherapy approach to address concomitant aspects of the vicious circle of myocardial ischemia (antioxidants, purine metabolism blockers/anti-inflammatory drugs)
-
Easy adoption and clinical penetration in the horizon.
2. Targeted Myocardial Restoration with Injectable Hydrogels
2.1. Post-MI Survivability
In the vast majority of cases, MI is a consequence of a vulnerable plaque rupture and a subsequent intracoronary thrombosis. The process initiates maladaptive changes in the myocardium, termed “cardiac remodeling“, which may result in the development of HF (Figure 1). The clinical sequelae are encountered in up to three-quarters of patients within five years after their first coronary event [3]. Importantly, HF has not only a significant impact on patients‘ functional capacity and quality of life, but the disease also significantly affects their life expectancy. Available data indicate that approximately half of the patients with HF do not survive for more than five years after the diagnosis [4], meaning that despite the advances in cardiac care, survival rates in this patient population are still very poor and are comparable to those observed in many types of cancer [5][6]. Given the above, more still needs to be done to tackle the burden of the disease more efficiently, thus triggering alternative mono- or poly-therapeutic treatments using viable matter and scaffolds.
2.2. Injectable Hydrogel-Based Approach for Cardiac Tissue Engineering
Owing to the intricate myocardial architecture and function, we believe that the triple approach (i.e., enhancing viability, counteracting inflammation, and stabilizing the diminishing architectural integrity of the left ventricle) yields the best restorative effect. Some of the most promising therapeutic compounds are hydrogel-based biomaterials that can not only provide mechanical support for a failing heart, but can also serve as a vehicle for cells, growth factors, and drugs. Because of their potential for minimally invasive transcatheter delivery, injectable hydrogels appear to be one of the most promising types of compounds in terms of their potential clinical application. Several types of hydrogel-based approaches for cardiac tissue repair have been investigated to date. Each category of hydrogels has its advantages and disadvantages that can influence their potential clinical applicability. There are various types of hydrogels with different properties based on their origin (natural/synthetic), various mechanisms of cross-linking, etc.
Based on the best evidence, we have observed a diverse range of compounds with none of the compositions showing clear superiority (Table 1). There are a number of studies, using acellular hydrogel by changing the matrix composition, more focused to investigate whether hydrogel characteristics (i.e, stiffening) enhances therapeutic efficacy to limit LV remodeling and heart failure [7]. Synthetic hydrogel: poly (NIPAAm-co-HEMA-co-MAPLA) (Sigma-Aldrich, St. Louis, MO, USA) was used in some studies [8][9], but hyaluronic acid-based hydrogel was used in most cases. Cell types, including skeletal myoblasts (SKMs), CMs, and other progenitor cells capable of differentiation to CMs, like embryonic stem cells (ESCs), ESC-derived CMs (ESCCMs), and mesenchymal stem cells (MSCs) with limited potentials, were investigated. Human umbilical mesenchymal stem cells [hUMSC] are a new focus [10], whereas basic fibroblast growth factor (bFGF), acidic gelatin hydrogel microspheres (AGHM), and vascular endothelial growth factor (VEGF) are in use with non-superiority to each other. Vu et al. used hyaluronic acid-based hydrogel coupled with PRP and showed improved host-cell viability [11]; while Traverse et al. [12] reported the first-in-human study with VentriGelTM (ECM from the decellularized porcine myocardium) in patients with 1st STEMI treated by PCI within a period of post-intervention between 60 days and 3 years, and found MRI evidence of LV remodeling and a clinical improvement in the study subgroup.
Table 1. Hydrogel characterization and mode of delivery.
| Author | Method of MI Creation | Artery Involved | Cell Delivered | Type of Matrix | Method of Delivery to Myocardium |
|---|---|---|---|---|---|
| Giordano, C et al., 2013 [13] | Left thoracotomy with ameroid constrictor | Proximal LCx | CAC | Type-I rat tail collagen cross-linked with glutaraldehyde (BD Bioscience, Oakville, Canada) | Open; Intramyocardial |
| Leor, J et al., 2009 [14] | Balloon occlusion | Mid-LAD artery | Acellular | Sodium alginate (VLVG, NovaMatrix, FMC Biopolymers, Drammen, Norway) | Intracoronary |
| Matsumura, Y et al., 2019 [8] | Left thoracotomy with suture ligation | Between 1st and 2nd diagonal branches | Acellular | Synthetic Hydrogel: Poly (NIPAAm-co-HEMA-co-MAPLA) (Sigma-Aldrich, USA) | Open; Intramyocardial |
| Qiang Wang et al., 2021 [10] | Left thoracotomy with suture ligation | LAD distal to origin of 2nd branch | hUMSC | Bovine collagen | Open; Intramyocardial |
| Yamamoto, T et al., 2001 [15] | Left thoracotomy with suture ligation | LAD between 1st and 2nd diagonal branches | bFGF | AGHM | Open; Subepicardial implantation |
| Zhou, D et al., 2012 [16] | Left thoracotomy with suture ligation | LAD below 1st diagonal branch | VEGF165 | Temperature-responsive Chitosan hydrogel | Open; Transmyocardial jet revascularization |
| Cohen, J.E et al., 2020 [17] | Left thoracotomy with suture ligation | 2nd and 3rd diagonal branches of LAD | NRG (R&D Systems, Minneapolis, MN, USA) | HEMA-HA based hydrogel (Lifecore Biomedical Inc., Chaska, MN, USA) | Open; intramyocardial |
| Contessotto, P et al., 2021 [18] | Left thoracotomy with suture ligation | LAD from 1st diagonal branch, moving distally till apex | Acellular | ELRs hydrogel | Open; Intramyocardial |
| Li, Y et al., 2021 [19] | Left thoracotomy with suture ligation | 1st two obtuse marginal arteries of LCx | MSN/miR-21-5p complex | Injectable hydrogel matrix | Open; Intramyocardial |
| Purcell, B.P et al., 2013 [20] | Left thoracotomy with suture ligation | 1st two obtuse marginal arteries of LCx | Full-length rTIMP-3 | Hyaluronic acid-based hydrogel with MMP | Open; Intramyocardial |
| Chang, M.Y et al., 2016 [21] | Left thoracotomy with suture ligation | Mid-LAD | CB-MNC | Hyaluronic acid hydrogel | Open; Intramyocardial |
| Ifkovits, J. L et al., 2010 [22] | Left thoracotomy with suture ligation | LAD and 2nd diagonal coronary artery | Acellular | Methacrylated hyaluronic acid macromers (MeHA) hydrogel | Open; Intramyocardial |
| Koudstaal, S et al., 2014 [23] | 75 min intracoronary balloon occlusion | LCx | IGF-1/HGF | UPy hydrogel | Open; Intramyocardial |
| Lin, Y.D et al., 2015 [24] | Left thoracotomy with suture ligation | Mid-LAD | MNCs | Peptide nanofibers | Open; Intramyocardial |
| Liu, Y et al., 2006 [25] | Left thoracotomy with suture ligation | LAD distal to 1st diagonal branch | bFGF, BDNF | Gelatin hydrogel (Boster Bioengineering Company, Wuhan, China) | Open; Intramyocardial |
| Yao Chang, M et al., 2005 [26] | Left thoracotomy with suture ligation | Mid-LAD | Bone marrow MNC | Peptide nanofibers | Open; Intramyocardial |
| Rodell, C.B et al., 2016 [7] | Left thoracotomy with suture ligation | Selective ligation of obtuse marginal branches | Acellular | Guest-host hydrogels; Dual-crosslinking hydrogels | Open; Intramyocardial |
| Spaulding, K.A et al., 2020 [9] | Left thoracotomy with suture ligation | LAD and its diagonal branches | Acellular | NIPAAm-PEG1500 hydrogel (Sigma-Aldrich, USA) | Open; Intramyocardial |
| Takehara, N et al., 2008 [27] | 90 min intracoronary balloon occlusion, followed by reperfusion | LAD | bFGF (Kaken Pharmaceutical Co., Tokyo, Japan) |
Gelatin hydrogel | Open; Intramyocardial |
| Vu, T.D et al., 2011 [11] | Left thoracotomy with suture ligation | Proximal LCx | Platelet-rich plasma | Hyaluronate Gelatin (Glycosan BioSystems Inc, Salt Lake City, UT, USA) | Open; Intramyocardial |
| Traverse, J.H et al., 2019 [12] | Patients with 1st STEMI treated by PCI within past 60 days to 3 years with moderate LV dysfunction. | − | Acellular | VentriGelTM—ECM from decellularized porcine myocardium | Transcatheter delivery through endocardium into myocardium |
LCx: Left Cirumflex Artery; CAC: Circulating Angiogenic Cells; LAD: Left Anterior Descending Artery; hUMSC: Human Umbilibal Mesenchymal Stem Cell; bFGF: Basic Fibroblast Growth Factor; AGHM: Acidic Gelatin Hydrogel Microspheres; VEGF: Vascular Endothelial Growth Factor; NRG: Neuregulin; HEMA-HA: Hyaluronic Acid Macromers with Hydroxyethyl Methacrylate Group Modification; ELRs: Elastin-like Recombinamers; MSN: Mesoporous Silica Nanoparticles; miR: microRNA; rTIMP-3: Tissue Inhibitors of Metalloproteinase Recombinant Protein; MMP: Matrix Metalloproteinase; CB-MNC: Cord Blood Mononuclear Cells; MeHA: Methacrylated Hyaluronic Acid; IGF-1: Insulin-like Growth Factor 1; HGF: Hepatocyte Growth Factor; UPy: Ureidopyrimidinone; MNCs: Mononuclear Cells; BDNF: Brain-Derived Neurotrophic Factor; NIPAAm: N-Isopropyl Acrylamide; PEG: Polyethylene Glycol.
2.3. Less Invasive Administration Modes
One of the most important aspects of hydrogel-based myocardial restoration therapy is the mode of delivery. In the context of the increasing role of minimally invasive techniques, a particular emphasis has been placed on shifting away from open heart surgery to catheter-based techniques. We doubt that any restoration method involving major surgical trauma can survive as a stand-alone treatment, as no patient, cardiologist, or surgeon will adopt it. Second, therapy may have to be chronic and repeated (i.e., multiple sessions during the process of time post-MI as HF chronifies). The patient cannot undergo countless re-dos if the procedure is invasive. This has prompted researchers to develop new devices for pinpointing the delivery of therapeutic compounds into a desired area of the myocardium. As a result, catheter-based techniques for myocardial restoration therapy have evolved from simple intracoronary injections (which are far from perfect, due to the rapid washout of an intravascular compound) to techniques with more efficient therapeutic retention. One of the examples is the TransAccess catheter system with fluoroscopic and intravascular ultrasound guidance, which was used for autologous skeletal myoblast delivery [28]. Currently, the most advanced device for intramyocardial delivery of therapeutic compounds is the NOGA system (Figure 2). The latter allows for the performance of 3D electromechanical mapping of the LV in order to identify target zones and perform precise transendocardial injections of therapeutics. Available data and the authors‘ own experience, derived from large animal models, confirm that the NOGA device is safe and highly effective.
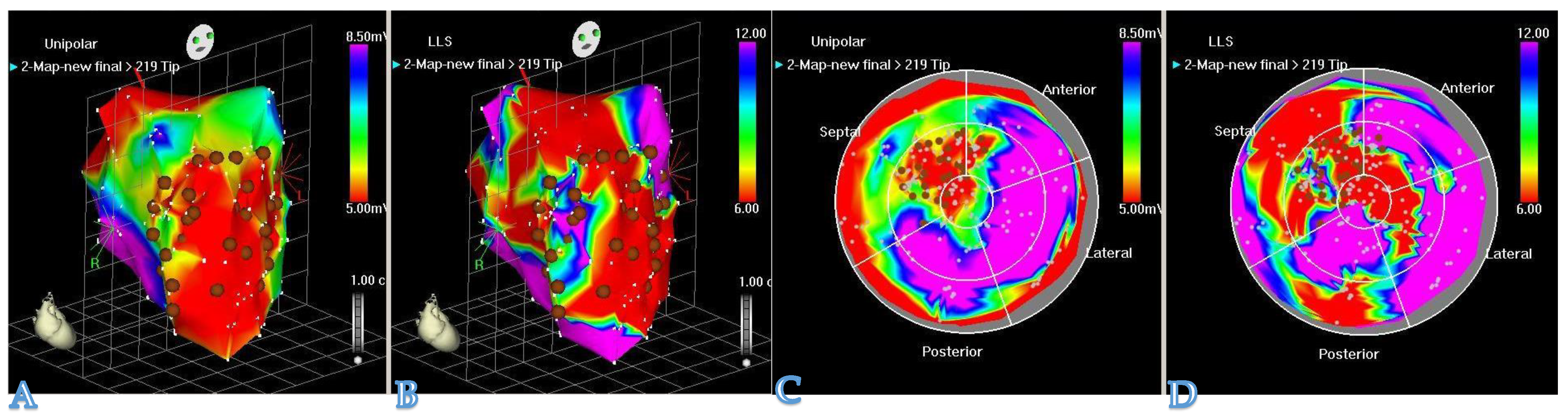 Figure 2. The NOGA® system allows visualization of the LV contraction in 3D. NOGA is able to map the heart in a full 360° rotation. (A,C): unipolar voltage maps (mV) and (B,D): regional wall motion maps by local linear shortening (LLS%) can help the electromechanical assessment of the myocardium. NOGA shows viability on the left column; dense scarring is visible at the apex and the antero-septal wall (red); scar area (<0.5 mV) = RED; viable tissue (>1.5 mV) = PURPLE. Comparing the bipolar and unipolar maps, NOGA is able to define border zone areas better.
Figure 2. The NOGA® system allows visualization of the LV contraction in 3D. NOGA is able to map the heart in a full 360° rotation. (A,C): unipolar voltage maps (mV) and (B,D): regional wall motion maps by local linear shortening (LLS%) can help the electromechanical assessment of the myocardium. NOGA shows viability on the left column; dense scarring is visible at the apex and the antero-septal wall (red); scar area (<0.5 mV) = RED; viable tissue (>1.5 mV) = PURPLE. Comparing the bipolar and unipolar maps, NOGA is able to define border zone areas better.3. Conclusions
Less Invasive procedures, coupled with injectable compounds, present a valid platform for a translational restoration protocol, which may be adopted by interventional cardiologists and heart surgeons. Polytherapeutic adjuvants, such as antioxidants, paracrine-active drugs, and anti-inflammatory substances, may be added to the protocols to ensure a sustained myocardial restoration effect.
Among all biomaterials currently used in cardiac tissue engineering, injectable hydrogels, with their potential for minimally invasive delivery and in-vivo breakdown into harmless derivatives, represent the most promising therapeutic option. However, the translational pathway from bench to bedside is challenging and still needs to be explored. It can be anticipated that in the next few decades the role of cell-vehicle compounds in the treatment of ischemic HF patients will expand, and injectable hydrogels will penetrate into the clinical arena to a higher extent.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9060595
References
- Menasche, P.; Alfieri, O. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200.
- Yu, H.; Lu, K. Stem cell therapy for ischemic heart diseases. Br. Med. Bull. 2017, 121, 135–154.
- Ezekowitz, J.A.; Kaul, P. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20.
- Barasa, A.; Schaufelberger, M. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 5–32.
- Mamas, M.A.; Sperrin, M. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104.
- Stewart, S.; Ekman, I. Population impact of heart failure and the most common forms of cancer: A study of 1 162 309 hospital cases in Sweden (1988 to 2004). Circ. Cardiovasc. Qual. Outcomes 2010, 3, 573–580.
- Rodell, C.B.; Lee, M.E. Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left-Ventricular Remodeling. Circ. Cardiovasc. Interv. 2016, 9, e004058.
- Matsumura, Y.; Zhu, Y. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289.
- Spaulding, K.A.; Zhu, Y. Myocardial injection of a thermoresponsive hydrogel with reactive oxygen species scavenger properties improves border zone contractility. J. Biomed. Mater. Res. A. 2020, 108, 1736–1746.
- Wang, Q.; He, X. Injectable collagen scaffold promotes swine myocardial infarction recovery by long-term local retention of transplanted human umbilical cord mesenchymal stem cells. Sci. China Life Sci. 2021, 64, 269–281.
- Vu, T.D.; Pal, S.N. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: A translational approach: Vu and Pal “Myocardial Repair: PRP, Hydrogel and Supplements”. Biomaterials 2015, 45, 27–35.
- Traverse, J.H.; Henry, T.D. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. Am. Coll. Cardiol. Basic Trans. Sci. 2019, 4, 659–669.
- Giordano, C.; Stephanie, L. Preclinical Evaluation of Biopolymer-Delivered Circulating Angiogenic Cells in a Swine Model of Hibernating Myocardium. Circ. Cardiovasc. Imaging 2013, 6, 982–991.
- Leor, J.; Tuvia, S. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023.
- Yamamoto, T.; Suto, N. Intramyocardial delivery of basic fibroblast growth factor-impregnated gelatin hydrogel microspheres enhances collateral circulation to infarcted canine myocardium. Jpn. Circ. J. 2001, 65, 439–444.
- Zhou, D.; Xiong, L. Effects of transmyocardial jet revascularization with chitosan hydrogel on channel patency and angiogenesis in canine infarcted hearts. J. Biomed. Mater. Res. Part A 2013, 101A, 567–574.
- Cohen, J.E.; Goldstone, A.B. A Bioengineered Neuregulin-Hydrogel Therapy Reduces Scar Size and Enhances Post-Infarct Ventricular Contractility in an Ovine Large Animal Model. J. Cardiovasc. Dev. Dis. 2020, 7, 53.
- Contessotto, P.; Orbanić, D. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 2021, 13, eaaz5380.
- Li, Y.; Chen, X. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, eabd6740.
- Purcell, B.P.; Barlow, S.C. Delivery of a matrix metalloproteinase-responsive hydrogel releasing TIMP-3 after myocardial infarction: Effects on left ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H814–H825.
- Chang, M.Y.; Huang, T.T. Injection of Human Cord Blood Cells With Hyaluronan Improves Postinfarction Cardiac Repair in Pigs. Stem Cells Transl. Med. 2016, 5, 56–66.
- Ifkovits, J.L.; Tous, E. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512.
- Koudstaal, S.; Bastings, M.M.C. Sustained Delivery of Insulin-Like Growth Factor-1/Hepatocyte Growth Factor Stimulates Endogenous Cardiac Repair in the Chronic Infarcted Pig Heart. J. Cardiovasc. Trans. Res. 2014, 7, 232–241.
- Li, Y.D.; Chang, M.Y. Injection of Peptide nanogels preserves postinfarct diastolic function and prolongs efficacy of cell therapy in pigs. Tissue Eng. Part A. 2015, 21, 1662–1671.
- Liu, Y.; Sun, L. Application of bFGF and BDNF to Improve Angiogenesis and Cardiac Function. J. Surg. Res. 2006, 136, 85–91.
- Chang, M.Y.; Chang, C.H. The time window for therapy with peptide nanofibers combined with autologous bone marrow cells in pigs after acute myocardial infarction. PLoS ONE 2015, 10, e0115430.
- Takehara, N.; Tsutsumi, Y. Controlled Delivery of Basic Fibroblast Growth Factor Promotes Human Cardiosphere-Derived Cell Engraftment to Enhance Cardiac Repair for Chronic Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1858–1865.
- Siminiak, T.; Fiszer, D. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: The POZNAN trial. Eur. Heart J. 2005, 26, 1188–1195.
This entry is offline, you can click here to edit this entry!
