G-protein coupled receptors (GPCRs) play important roles in cell biology and insects’ physiological processes, toxicological response and the development of insecticide resistance. New information on genome sequences, proteomic and transcriptome analysis and expression patterns of GPCRs in organs such as the central nervous system in different organisms has shown the importance of these signaling regulatory GPCRs and their impact on vital cell functions. Our growing understanding of the role played by GPCRs at the cellular, genome, transcriptome and tissue levels is now being utilized to develop new targets that will sidestep many of the problems currently hindering human disease control and insect pest management.
- G-protein coupled receptor regulation pathway
- GPCR physiological functions
- tissue specific expression
- genome sequences analysis
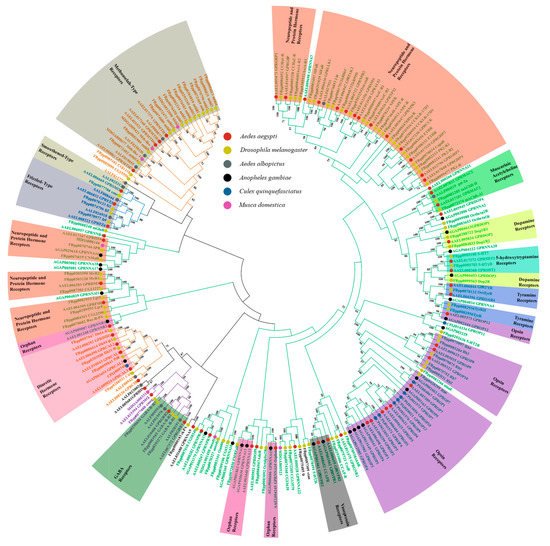
- phylogenic tree
- insect physiology
- insecticide resistance
1. Introduction
2. Whole Genome Sequencing and Transcriptome Analysis—Sequence Comparison and GPCR Characterization in Insects
2.1. Classification Systems Used in Characterizing GPCRs
| Insect Species | Total Number of Genes | Class A Gene Number |
Class B Gene Number | Class C Gene Number | Class F Gene Number | Source of Genome Information | Reference |
|---|---|---|---|---|---|---|---|
| D. melanogaster | ~200 | >70 | ~20 | ~5 | ~5 | https://flybase.org/ (accessed on 7 May 2021) |
[84][85] |
| An. gambiae | ~276 | 81 | 21 | 8 | ~8 | https://www.ncbi.nlm.nih.gov/genome/46?genome_assembly_id=22679 (accessed on 7 May 2021) |
[86] |
| Ae. aegypti | 135 | 89 | 24 | 8 | 11 | https://www.ncbi.nlm.nih.gov/genome/44?genome_assembly_id=322291 (accessed on 7 May 2021) |
[80] |
| B. mori | ~90 | ~70 | ~7 | ~8 | ~4 | https://www.ncbi.nlm.nih.gov/genome/76?genome_assembly_id=1491718 (accessed on 7 May 2021) |
[87] |
| A. mellifera | ~50 | ~31 | ~4 | Not clear | Not clear | https://www.ncbi.nlm.nih.gov/genome/48?genome_assembly_id=403979 (accessed on 7 May 2021) |
[17] |
| L. cuprina | 197 | 73 | 18 | 9 | Not clear | https://www.ncbi.nlm.nih.gov/genome/12732?genome_assembly_id=358015 (accessed on 7 May 2021) |
[88] |
| Cx. quinquefasciatus | 115 | 52 | 4 | Not clear | Not clear | https://www.ncbi.nlm.nih.gov/genome/393?genome_assembly_id=1502880 (accessed on 7 May 2021) |
[42] |
| M. domestica | 94 | 55 | 27 | 4 | Not clear | https://www.ncbi.nlm.nih.gov/genome/14461?genome_assembly_id=44793 (accessed on 7 May 2021) |
[72] |
2.2. Receptors of GPCR Involved in Insect Physiology or Insecticide Resistance That Are Potential Targets for Insecticide Development
| Receptor Group | Receptor Name | Classes | Species | Function | Reference |
|---|---|---|---|---|---|
| 5-HT receptors | Trica5-HT7 R | Class A | Tribolium castaneum | Insect’s neural processes | [134] |
| Adipokinetic hormone receptor | Akh receptor | Class A | Bactrocera dorsalis | Lipid mobilization | [62] |
| Adipokinetic hormone receptor | Akh receptor | Class A | D. melanogaster | Lipid mobilization | [51] |
| Adipokinetic hormone receptor | Akh receptor | Class A | Nilaparvata lugens | Lipid mobilization | [53] |
| Allatostatin receptor | AstAR1 | Class A | D. melanogaster | Metamorphosis | [98] |
| Allatostatin receptor | DAR-1/DAR-2 | Class A | D. melanogaster | Feeding modulation | [99] |
| Allatostatin receptor | Dippu-AstR | Class A | Diploptera punctata | Juvenile hormone synthesis | [128] |
| Arginine vasopressin-like receptor | AVPL receptor | Class A | T. castaneum | Diuretic signaling pathway | [92] |
| Calcitonin receptors | GPCRCAL1 | Class A | Ae. aegypti | primary urine secretion | [66] |
| CCHa2 receptor | CCHa2-R | Class A | D. melanogaster | Insulin production | [105] |
| Diapause hormone receptor | DH-R | Class A | Ae. aegypti | Development | [39] |
| Diapause hormone receptor | Bommo-DHR | Class A | B. mori | Development | [45] |
| Diapause hormone receptor | HzDHr | Class A | Helicoverpa zea | Development | [41] |
| Dopamine receptor | Dop1R2, DmDopEcR | Class A | D. melanogaster | Morphogenesis | [34][97] |
| Dopamine receptor | DopEcR | Class A | D. melanogaster | Mushroom and locomotor activity | [127] |
| Dopamine receptor | DopEcR | Class A | D. melanogaster | Ethanol-induced sedation | [68] |
| Dopamine receptor | AipsDopEcR | Class A | Agrotis ipsilon | Sexual activity regulation | [60][126] |
| Dopamine receptor | DopEcR | Class A | Helicoverpa armigera | Morphogenesis | [35] |
| Dopamine receptor | D2R | Class A | T. castaneum | Morphogenesis | [29] |
| Leucokinin receptor | LKr | Class A | D. melanogaster | Feeding modulation | [100][103] |
| Myosuppressin receptors | CG8985/CG13803 | Class A | D. melanogaster | visceral muscles inhibition | [108] |
| Neuropeptide receptors | GPCR-B2 | Class A | B. mori | Ecdysone synthesis | [33] |
| Neuropeptide receptors | Schgr-sNPFR | Class A | Schistocerca gregaria | Feeding behavior | [50] |
| Neuropeptide Drosulfakinin receptor | CCKLR-17D1 | Class A | D. melanogaster | Fighting behavior | [104] |
| Orphan receptor | DLGR2 | Class A | D. melanogaster | Bursicon bioactivity | [25] |
| Orphan receptor | BNGR-A4 receptor | Class A | B. mori | Food intake and growth | [130] |
| Rhodopsin receptors | Rh2 | Class A | T. castaneum | Reproduction | [22] |
| Sex peptide receptor | SPR | Class A | D. melanogaster | Reproductive behavior | [101] |
| SIFamide receptor | SIFaR | Class A | D. melanogaster | Reproductive behavior | [102] |
| Tyramine receptor | TAR1 | Class A | Rhipicephalus (Boophilus) microplus | Development of antiparasitic | [131] |
| Corticotropin releasing factor receptor | CG12370 | Class B | D. melanogaster | Water balance | [113] |
| Diuretic hormone receptors | DH31R | Class B | D. melanogaster | temperature regulation and homeostasis | [114] |
| Methuselah receptor | mth | Class B | D. melanogaster | Oxidative stress resistance | [136] |
| Methuselah receptor | Ldmthl1 | Class B | Lymantria dispar | Insect longevity | [135] |
| Metabotropic GABA receptors | D-GABABR1, R2 and R3 | Class C | D. melanogaster | Central nervous system | [118] |
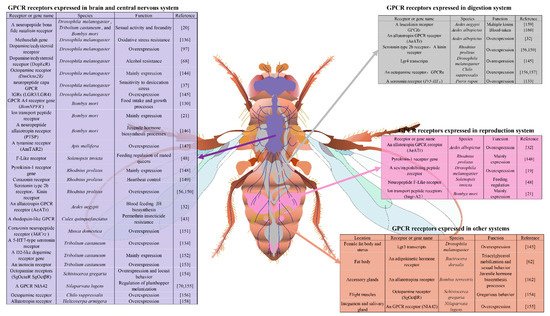
3. Tissue Specific Expression Analysis of GPCRs in Insects
3.1. Brain Tissue and Central Nervous System
3.2. Digestion and Reproduction Systems
3.3. Other Insect Organs
This entry is adapted from the peer-reviewed paper 10.3390/molecules26102993
References
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12.
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729.
- Maudsley, S.; Martin, B.; Luttrell, L.M. The Origins of Diversity and Specificity in G Protein-Coupled Receptor Signaling. J. Pharmacol. Exp. Ther. 2005, 314, 485–494.
- Lagerström, M.C.; Schiöth, H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 339–357.
- Spehr, M.; Munger, S.D. Olfactory receptors: G protein-coupled receptors and beyond. J. Neurochem. 2009, 109, 1570–1583.
- Millar, R.P.; Newton, C.L. The Year in G Protein-Coupled Receptor Research. Mol. Endocrinol. 2010, 24, 261–274.
- Eglen, R.M.; Reisine, T. GPCRs Revisited: New Insights Lead to Novel Drugs. Pharmaceuticals 2011, 4, 244–272.
- Gether, U. Uncovering Molecular Mechanisms Involved in Activation of G Protein-Coupled Receptors. Endocr. Rev. 2000, 21, 90–113.
- Goldsmith, Z.G.; Dhanasekaran, D.N. G Protein regulation of MAPK networks. Oncogene 2007, 26, 3122–3142.
- Gerald, W.Z. Calcium channel signaling complexes with receptors and channels. Curr. Mol. Pharmacol. 2015, 8, 8–11.
- Flower, D.R. Modelling G-protein-coupled receptors for drug design. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1999, 1422, 207–234.
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730.
- Robas, N.; O’Reilly, M.; Katugampola, S.; Fidock, M. Maximizing serendipity: Strategies for identifying ligands for orphan G-protein-coupled receptors. Curr. Opin. Pharmacol. 2003, 3, 121–126.
- Jacoby, E.; Bouhelal, R.; Gerspacher, M.; Seuwen, K. The 7 TM G-Protein-Coupled Receptor Target Family. ChemMedChem 2006, 1, 760–782.
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842.
- Congreve, M.; de Graaf, C.; Swain, N.A.; Tate, C.G. Impact of GPCR Structures on Drug Discovery. Cell 2020, 181, 81–91.
- Hauser, F.; Cazzamali, G.; Williamson, M.; Blenau, W.; Grimmelikhuijzen, C.J. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog. Neurobiol. 2006, 80, 1–19.
- Hauser, F.; Cazzamali, G.; Williamson, M.; Park, Y.; Li, B.; Tanaka, Y.; Predel, R.; Neupert, S.; Schachtner, J.; Verleyen, P.; et al. A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Front. Neuroendocr. 2008, 29, 142–165.
- Poels, J.; Van Loy, T.; Vandersmissen, H.P.; Van Hiel, B.; Van Soest, S.; Nachman, R.J.; Broeck, J.V. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell. Mol. Life Sci. 2010, 67, 3511–3522.
- Jiang, H.; Lkhagva, A.; Park, Y.; Kim, Y.-J.; Daubnerová, I.; Chae, H.-S.; Šimo, L.; Jung, S.-H.; Yoon, Y.-K.; Lee, N.-R.; et al. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc. Natl. Acad. Sci. USA 2013, 110, E3526–E3534.
- Nagai, C.; Mabashi-Asazuma, H.; Nagasawa, H.; Nagata, S. Identification and Characterization of Receptors for Ion Transport Peptide (ITP) and ITP-like (ITPL) in the Silkworm Bombyx mori. J. Biol. Chem. 2014, 289, 32166–32177.
- Bai, H.; Palli, S.R. Identification of G protein-coupled receptors required for vitellogenin uptake into the oocytes of the red flour beetle, Tribolium castaneum. Sci. Rep. 2016, 6, 27648.
- Jing, Y.-P.; An, H.; Zhang, S.; Wang, N.; Zhou, S. Protein kinase C mediates juvenile hormone–dependent phosphorylation of Na+/K+-ATPase to induce ovarian follicular patency for yolk protein uptake. J. Biol. Chem. 2018, 293, 20112–20122.
- Marciniak, P.; Urbański, A.; Lubawy, J.; Szymczak, M.; Pacholska-Bogalska, J.; Chowański, S.; Kuczer, M.; Rosiński, G. Short neuropeptide F signaling regulates functioning of male reproductive system in Tenebrio molitor beetle. J. Comp. Physiol. B 2020, 190, 521–534.
- Mendive, F.M.; Van Loy, T.; Claeysen, S.; Poels, J.; Williamson, M.; Hauser, F.; Grimmelikhuijzen, C.J.; Vassart, G.; Broeck, J.V. Drosophila molting neurohormone bursicon is a heterodimer and the natural agonist of the orphan receptor DLGR2. FEBS Lett. 2005, 579, 2171–2176.
- Kim, Y.-J.; Zitnan, D.; Cho, K.-H.; Schooley, D.A.; Mizoguchi, A.; Adams, M.E. Central peptidergic ensembles associated with organization of an innate behavior. Proc. Natl. Acad. Sci. USA 2006, 103, 14211–14216.
- Žitňan, D.; Kim, Y.-J.; Žitňanová, I.; Roller, L.; Adams, M. Complex steroid–peptide–receptor cascade controls insect ecdysis. Gen. Comp. Endocrinol. 2007, 153, 88–96.
- Yamanaka, N.; Hua, Y.-J.; Roller, L.; Spalovská-Valachová, I.; Mizoguchi, A.; Kataoka, H.; Tanaka, Y. Bombyx prothoracicostatic peptides activate the sex peptide receptor to regulate ecdysteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 2060–2065.
- Bai, H.; Zhu, F.; Shah, K.; Palli, S.R. Large-scale RNAi screen of G protein-coupled receptors involved in larval growth, molting and metamorphosis in the red flour beetle. BMC Genom. 2011, 12, 388.
- Li, B.; Beeman, R.W.; Park, Y. Functions of duplicated genes encoding CCAP receptors in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 2011, 57, 1190–1197.
- Li, K.; Jia, Q.; Li, S. Juvenile hormone signaling—A mini review. Insect Sci. 2019, 26, 600–606.
- Nouzova, M.; Brockhoff, A.; Mayoral, J.G.; Goodwin, M.; Meyerhof, W.; Noriega, F.G. Functional characterization of an allatotropin receptor expressed in the corpora allata of Mosquitoes. Peptides 2012, 34, 201–208.
- Iga, M.; Nakaoka, T.; Suzuki, Y.; Kataoka, H. Pigment Dispersing Factor Regulates Ecdysone Biosynthesis via Bombyx Neuropeptide G Protein Coupled Receptor-B2 in the Prothoracic Glands of Bombyx mori. PLoS ONE 2014, 9, e103239.
- Regna, K.; Kurshan, P.T.; Harwood, B.N.; Jenkins, A.M.; Lai, C.-Q.; Muskavitch, M.A.; Kopin, A.S.; Draper, I. A critical role for the Drosophila dopamine D1-like receptor Dop1R2 at the onset of metamorphosis. BMC Dev. Biol. 2016, 16, 15.
- Kang, X.-L.; Zhang, J.-Y.; Wang, D.; Zhao, Y.-M.; Han, X.-L.; Wang, J.-X.; Zhao, X.-F. The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet. 2019, 15, e1008331.
- Homma, T.; Watanabe, K.; Tsurumaru, S.; Kataoka, H.; Imai, K.; Kamba, M.; Niimi, T.; Yamashita, O.; Yaginuma, T. G protein-coupled receptor for diapause hormone, an inducer of Bombyx embryonic diapause. Biochem. Biophys. Res. Commun. 2006, 344, 386–393.
- Terhzaz, S.; Cabrero, P.; Robben, J.H.; Radford, J.C.; Hudson, B.D.; Milligan, G.; Dow, J.A.T.; Davies, S.-A. Mechanism and Function of Drosophila capa GPCR: A Desiccation Stress-Responsive Receptor with Functional Homology to Human NeuromedinU Receptor. PLoS ONE 2012, 7, e29897.
- Bryon, A.; Wybouw, N.; Dermauw, W.; Tirry, L.; Van Leeuwen, T. Genome wide gene-expression analysis of facultative reproductive diapause in the two-spotted spider mite Tetranychus urticae. BMC Genom. 2013, 14, 1–20.
- Choi, M.-Y.; Estep, A.; Sanscrainte, N.; Becnel, J.; Meer, R.K.V. Identification and expression of PBAN/diapause hormone and GPCRs from Aedes aegypti. Mol. Cell. Endocrinol. 2013, 375, 113–120.
- Devambez, I.; Agha, M.A.; Mitri, C.; Bockaert, J.; Parmentier, M.-L.; Marion-Poll, F.; Grau, Y.; Soustelle, L. Gαo Is Required for L-Canavanine Detection in Drosophila. PLoS ONE 2013, 8, e63484.
- Jiang, H.; Wei, Z.; Nachman, R.J.; Park, Y. Molecular cloning and functional characterization of the diapause hormone receptor in the corn earworm Helicoverpa zea. Peptides 2014, 53, 243–249.
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of G-protein-coupled Receptor-related Genes in Insecticide Resistance of the Mosquito, Culex quinquefasciatus. Sci. Rep. 2015, 4, 6474.
- Li, T.; Cao, C.; Yang, T.; Zhang, L.; He, L.; Xi, Z.; Bian, G.; Liu, N. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in Mosquitoes, Culex quinquefasciatus. Sci. Rep. 2015, 5, 17772.
- Li, T.; Liu, N. Regulation of P450-mediated permethrin resistance in Culex quinquefasciatus by the GPCR/Gαs/AC/cAMP/PKA signaling cascade. Biochem. Biophys. Rep. 2017, 12, 12–19.
- Shen, Z.; Jiang, X.; Yan, L.; Chen, Y.; Wang, W.; Shi, Y.; Shi, L.; Liu, D.; Zhou, N. Structural basis for the interaction of diapause hormone with its receptor in the silkworm, Bombyx mori. FASEB J. 2018, 32, 1338–1353.
- Chen, C.-H.; Di, Y.-Q.; Shen, Q.-Y.; Wang, J.-X.; Zhao, X.-F. The steroid hormone 20-hydroxyecdysone induces phosphorylation and aggregation of stromal interacting molecule 1 for store-operated calcium entry. J. Biol. Chem. 2019, 294, 14922–14936.
- Petruccelli, E.; Lark, A.; Mrkvicka, J.A.; Kitamoto, T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J. Neurogenet. 2020, 34, 55–68.
- Chen, M.-E.; Pietrantonio, P.V. The short neuropeptide F-like receptor from the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Arch. Insect Biochem. Physiol. 2006, 61, 195–208.
- Kersch, C.N.; Pietrantonio, P.V. MosquitoAedes aegypti (L.) leucokinin receptor is critical forin vivofluid excretion post blood feeding. FEBS Lett. 2011, 585, 3507–3512.
- Dillen, S.; Zels, S.; Verlinden, H.; Spit, J.; Van Wielendaele, P.; Broeck, J.V. Functional Characterization of the Short Neuropeptide F Receptor in the Desert Locust, Schistocerca gregaria. PLoS ONE 2013, 8, e53604.
- Baumbach, J.; Xu, Y.; Hehlert, P.; Kühnlein, R.P. Gαq, Gγ1 and Plc21C Control Drosophila Body Fat Storage. J. Genet. Genom. 2014, 41, 283–292.
- Lin, F.; Hossain, M.A.; Post, S.; Karashchuk, G.; Tatar, M.; De Meyts, P.; Wade, J.D. Total Solid-Phase Synthesis of Biologically Active Drosophila Insulin-Like Peptide 2 (DILP2). Aust. J. Chem. 2017, 70, 208–212.
- Lu, K.; Zhang, X.; Chen, X.; Li, Y.; Li, W.; Cheng, Y.; Zhou, J.; You, K.; Zhou, Q. Adipokinetic Hormone Receptor Mediates Lipid Mobilization to Regulate Starvation Resistance in the Brown Planthopper, Nilaparvata lugens. Front. Physiol. 2018, 9, 1730.
- Marchal, E.; Schellens, S.; Monjon, E.; Bruyninckx, E.; Marco, H.G.; Gäde, G.; Broeck, J.V.; Verlinden, H. Analysis of Peptide Ligand Specificity of Different Insect Adipokinetic Hormone Receptors. Int. J. Mol. Sci. 2018, 19, 542.
- Yang, H.; Huang, J.; Liu, Y.; Li, J.; Luo, S.; Wu, J. Prediction of the post-translational modifications of adipokinetic hormone receptors from solitary to eusocial bees. Sociobiology 2018, 65, 271–279.
- Sangha, V.; Lange, A.B.; Orchard, I. Identification and cloning of the kinin receptor in the Chagas disease vector, Rhodnius prolixus. Gen. Comp. Endocrinol. 2020, 289, 113380.
- Bainton, R.J.; Tsai, L.T.-Y.; Schwabe, T.; DeSalvo, M.; Gaul, U.; Heberlein, U. moody Encodes Two GPCRs that Regulate Cocaine Behaviors and Blood-Brain Barrier Permeability in Drosophila. Cell 2005, 123, 145–156.
- Hyun, S.; Lee, Y.; Hong, S.-T.; Bang, S.; Paik, D.; Kang, J.; Shin, J.; Lee, J.; Jeon, K.; Hwang, S.; et al. Drosophila GPCR Han Is a Receptor for the Circadian Clock Neuropeptide PDF. Neuron 2005, 48, 267–278.
- Thamm, M.; Balfanz, S.; Scheiner, R.; Baumann, A.; Blenau, W. Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell. Mol. Life Sci. 2010, 67, 2467–2479.
- Abrieux, A.; Debernard, S.; Maria, A.; Gaertner, C.; Anton, S.; Gadenne, C.; Duportets, L. Involvement of the G-Protein-Coupled Dopamine/Ecdysteroid Receptor DopEcR in the Behavioral Response to Sex Pheromone in an Insect. PLoS ONE 2013, 8, e72785.
- Kwon, H.; Agha, M.A.; Smith, R.C.; Nachman, R.J.; Marion-Poll, F.; Pietrantonio, P.V. Leucokinin mimetic elicits aversive behavior in Mosquito Aedes aegypti (L.) and inhibits the sugar taste neuron. Proc. Natl. Acad. Sci. USA 2016, 113, 6880–6885.
- Hou, Q.-L.; Chen, E.-H.; Jiang, H.-B.; Wei, D.-D.; Gui, S.-H.; Wang, J.-J.; Smagghe, G. Adipokinetic hormone receptor gene identification and its role in triacylglycerol mobilization and sexual behavior in the oriental fruit fly (Bactrocera dorsalis). Insect Biochem. Mol. Biol. 2017, 90, 1–13.
- Grohmann, L.; Blenau, W.; Erber, J.; Ebert, P.R.; Strünker, T.; Baumann, A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J. Neurochem. 2003, 86, 725–735.
- Johnson, E.C.; Bohn, L.M.; Taghert, P.H. Drosophila CG8422 encodes a functional diuretic hormone receptor. J. Exp. Biol. 2004, 207, 743–748.
- Kwon, H.; Lu, H.-L.; Longnecker, M.T.; Pietrantonio, P.V. Role in Diuresis of a Calcitonin Receptor (GPRCAL1) Expressed in a Distal-Proximal Gradient in Renal Organs of the Mosquito Aedes aegypti (L.). PLoS ONE 2012, 7, e50374.
- Kwon, H.; Pietrantonio, P.V. Calcitonin receptor 1 (AedaeGPCRCAL1) hindgut expression and direct role in myotropic action in females of the Mosquito Aedes aegypti (L.). Insect Biochem. Mol. Biol. 2013, 43, 588–593.
- Lee, D.; Broeck, J.V.; Lange, A.B. Identification and Expression of the CCAP Receptor in the Chagas’ Disease Vector, Rhodnius prolixus, and Its Involvement in Cardiac Control. PLoS ONE 2013, 8, e68897.
- Petruccelli, E.; Li, Q.; Rao, Y.; Kitamoto, T. The Unique Dopamine/Ecdysteroid Receptor Modulates Ethanol-Induced Sedation in Drosophila. J. Neurosci. 2016, 36, 4647–4657.
- Pietrantonio, P.V.; Xiong, C.; Nachman, R.J.; Shen, Y. G protein-coupled receptors in arthropod vectors: Omics and pharmacological approaches to elucidate ligand-receptor interactions and novel organismal functions. Curr. Opin. Insect Sci. 2018, 29, 12–20.
- Uchiyama, H.; Maehara, S.; Ohta, H.; Seki, T.; Tanaka, Y. Elevenin regulates the body color through a G protein-coupled receptor NlA42 in the brown planthopper Nilaparvata lugens. Gen. Comp. Endocrinol. 2018, 258, 33–38.
- Li, M.; Reid, W.R.; Zhang, L.; Scott, J.G.; Gao, X.; Kristensen, M.; Liu, N. A whole transcriptomal linkage analysis of gene co-regulation in insecticide resistant house flies, Musca domestica. BMC Genom. 2013, 14, 803.
- Ma, Z.; Zhang, Y.; You, C.; Zeng, X.; Gao, X. The role of G protein-coupled receptor-related genes in cytochrome P450-mediated resistance of the house fly, Musca domestica (Diptera: Muscidae), to imidacloprid. Insect Mol. Biol. 2019, 29, 92–103.
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Ѐntomol. 2015, 60, 537–559.
- Attwood, T.K.; Findlay, J.B.C. Fingerprinting G-protein-coupled receptors. Protein Eng. Des. Sel. 1994, 7, 195–203.
- Kolakowski, L.F., Jr. GCRDb: A G-protein-coupled receptor database. Recept. Channels 1994, 2, 1–7.
- Schiöth, H.B.; Fredriksson, R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 2005, 142, 94–101.
- Hu, G.-M.; Mai, T.-L.; Chen, C.-M. Visualizing the GPCR Network: Classification and Evolution. Sci. Rep. 2017, 7, 1–15.
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195.
- Holt, R.A.; Subramanian, G.M.; Halpern, A.; Sutton, G.G.; Charlab, R.; Nusskern, D.R.; Wincker, P.; Clark, A.G.; Ribeiro, J.C.; Wides, R.; et al. The Genome Sequence of the Malaria Mosquito Anopheles gambiae. Science 2002, 298, 129–149.
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome Sequence of Aedes aegypti, a Major Arbovirus Vector. Science 2007, 316, 1718–1723.
- Arensburger, P.; Megy, K.; Waterhouse, R.M.; Abrudan, J.; Amedeo, P.; Antelo, B.; Bartholomay, L.; Bidwell, S.; Caler, E.; Camara, F.; et al. Sequencing of Culex quinquefasciatus Establishes a Platform for Mosquito Comparative Genomics. Science 2010, 330, 86–88.
- Scott, J.G.; Warren, W.C.; Beukeboom, L.W.; Bopp, D.; Clark, A.G.; Giers, S.D.; Hediger, M.; Jones, A.K.; Kasai, S.; A Leichter, C.; et al. Genome of the house fly, Musca domestica L., a global vector of diseases with adaptations to a septic environment. Genome Biol. 2014, 15, 1–17.
- Li, F.; Zhao, X.; Li, M.; He, K.; Huang, C.; Zhou, Y.; Li, Z.; Walters, J.R. Insect genomes: Progress and challenges. Insect Mol. Biol. 2019, 28, 739–758.
- Brody, T.; Cravchik, A. Drosophila melanogaster G Protein–Coupled Receptors. J. Cell Biol. 2000, 150, F83–F88.
- Hanlon, C.D.; Andrew, D.J. Outside-in signaling–A brief review of GPCR signaling with a focus on the Drosophila GPCR family. J. Cell Sci. 2015, 128, 3533–3542.
- Hill, C.A.; Fox, A.N.; Pitts, R.J.; Kent, L.B.; Tan, P.L.; Chrystal, M.A.; Cravchik, A.; Collins, F.H.; Robertson, H.M.; Zwiebel, L.J. G Protein-Coupled Receptors inAnopheles gambiae. Science 2002, 298, 176–178.
- Fan, Y.; Sun, P.; Wang, Y.; He, X.; Deng, X.; Chen, X.; Zhang, G.; Chen, X.; Zhou, N. The G protein-coupled receptors in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 581–591.
- Anstead, C.A.; Korhonen, P.K.; Young, N.D.; Hall, R.S.; Jex, A.R.; Murali, S.C.; Hughes, D.S.; Lee, S.F.; Perry, T.; Stroehlein, A.J.; et al. Lucilia cuprina genome unlocks parasitic fly biology to underpin future interventions. Nat. Commun. 2015, 6, 7344.
- Şahbaz, B.D. Prediction and expression analysis of G protein-coupled receptors in the laboratory stick insect, Carausius morosus. Turk. J. Boil. 2019, 43, 77–88.
- Calkins, T.L.; Tamborindeguy, C.; Pietrantonio, P.V. GPCR annotation, G proteins, and transcriptomics of fire ant (Solenopsis invicta) queen and worker brain: An improved view of signaling in an invasive superorganism. Gen. Comp. Endocrinol. 2019, 278, 89–103.
- Veenstra, J.A.; Rombauts, S.; Grbić, M. In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem. Mol. Biol. 2012, 42, 277–295.
- Aikins, M.J.; Schooley, D.A.; Begum, K.; Detheux, M.; Beeman, R.W.; Park, Y. Vasopressin-like peptide and its receptor function in an indirect diuretic signaling pathway in the red flour beetle. Insect Biochem. Mol. Biol. 2008, 38, 740–748.
- Caers, J.; Verlinden, H.; Zels, S.; Vandersmissen, H.P.; Vuerinckx, K.; Schoofs, L. More than two decades of research on insect neuropeptide GPCRs: An overview. Front. Endocrinol. 2012, 3, 151.
- Garczynski, S.F.; Brown, M.R.; Shen, P.; Murray, T.F.; Crim, J.W. Characterization of a functional neuropeptide F receptor from Drosophila melanogaster. Peptides 2002, 23, 773–780.
- Vogel, K.J.; Brown, M.R.; Strand, M.R. Phylogenetic Investigation of Peptide Hormone and Growth Factor Receptors in Five Dipteran Genomes. Front. Endocrinol. 2013, 4, 193.
- Xia, R.; Li, M.; Wu, Y.; Qi, Y.; Ye, G.; Huang, J. A new family of insect muscarinic acetylcholine receptors. Insect Mol. Biol. 2016, 25, 362–369.
- Srivastava, D.P.; Yu, E.J.; Kennedy, K.; Chatwin, H.; Reale, V.; Hamon, M.; Smith, T.; Evans, P.D. Rapid, Nongenomic Responses to Ecdysteroids and Catecholamines Mediated by a Novel Drosophila G-Protein-Coupled Receptor. J. Neurosci. 2005, 25, 6145–6155.
- Deveci, D.; Martin, F.A.; Leopold, P.; Romero, N.M. AstA Signaling Functions as an Evolutionary Conserved Mechanism Timing Juvenile to Adult Transition. Curr. Biol. 2019, 29, 813–822.
- Chen, J.; Reiher, W.; Hermann-Luibl, C.; Sellami, A.; Cognigni, P.; Kondo, S.; Helfrich-Förster, C.; Veenstra, J.A.; Wegener, C. Allatostatin A Signalling in Drosophila Regulates Feeding and Sleep and Is Modulated by PDF. PLoS Genet. 2016, 12, e1006346.
- Zandawala, M.; Yurgel, M.E.; Texada, M.J.; Liao, S.; Rewitz, K.F.; Keene, A.C.; Nässel, D.R. Modulation of Drosophila post-feeding physiology and behavior by the neuropeptide leucokinin. PLoS Genet. 2018, 14, e1007767.
- Yapici, N.; Kim, Y.-J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nat. Cell Biol. 2007, 451, 33–37.
- Sellami, A.; Veenstra, J.A. SIFamide acts on fruitless neurons to modulate sexual behavior in Drosophila melanogaster. Peptides 2015, 74, 50–56.
- Yurgel, M.E.; Kakad, P.; Zandawala, M.; Nässel, D.R.; Godenschwege, T.A.; Keene, A.C. A single pair of leucokinin neurons are modulated by feeding state and regulate sleep–metabolism interactions. PLoS Biol. 2019, 17, e2006409.
- Wu, F.; Deng, B.; Xiao, N.; Wang, T.; Li, Y.; Wang, R.; Shi, K.; Luo, D.-G.; Rao, Y.; Zhou, C. A neuropeptide regulates fighting behavior in Drosophila melanogaster. eLife 2020, 9.
- Sano, H.; Nakamura, A.; Texada, M.J.; Truman, J.W.; Ishimoto, H.; Kamikouchi, A.; Nibu, Y.; Kume, K.; Ida, T.; Kojima, M. The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of Drosophila melanogaster. PLoS Genet. 2015, 11, e1005209.
- Iyison, N.B.; Sinmaz, M.G.; Sahbaz, B.D.; Shahraki, A.; Aksoydan, B.; Durdagi, S. In silico characterization of adipokinetic hormone receptor and screening for pesticide candidates against stick insect, Carausius morosus. J. Mol. Graph. Model. 2020, 101, 107720.
- Posnien, N.; Hopfen, C.; Hilbrant, M.; Ramos-Womack, M.; Murat, S.; Schönauer, A.; Herbert, S.L.; Nunes, M.D.S.; Arif, S.; Breuker, C.J.; et al. Evolution of Eye Morphology and Rhodopsin Expression in the Drosophila melanogaster Species Subgroup. PLoS ONE 2012, 7, e37346.
- Egerod, K.; Reynisson, E.; Hauser, F.; Cazzamali, G.; Williamson, M.; Grimmelikhuijzen, C.J.P. Molecular cloning and functional expression of the first two specific insect myosuppressin receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 9808–9813.
- Hauser, F.; Williamson, M.; Cazzamali, G.; Grimmelikhuijzen, C.J.P. Identifying neuropeptide and protein hormone receptors in Drosophila melanogaster by exploiting genomic data. Briefings Funct. Genom. Proteom. 2006, 4, 321–330.
- Bayliss, A.; Roselli, G.; Evans, P.D. A comparison of the signalling properties of two tyramine receptors from Drosophila. J. Neurochem. 2013, 125, 37–48.
- Li, C.; Chen, M.; Sang, M.; Liu, X.; Wu, W.; Li, B. Comparative genomic analysis and evolution of family-B G protein-coupled receptors from six model insect species. Gene 2013, 519, 1–12.
- De Mendoza, A.; Jones, J.W.; Friedrich, M. Methuselah/Methuselah-like G protein-coupled receptors constitute an ancient metazoan gene family. Sci. Rep. 2016, 6, 21801.
- Hector, C.E.; Bretz, C.A.; Zhao, Y.; Johnson, E.C. Functional differences between two CRF-related diuretic hormone receptors in Drosophila. J. Exp. Biol. 2009, 212, 3142–3147.
- Goda, T.; Doi, M.; Umezaki, Y.; Murai, I.; Shimatani, H.; Chu, M.L.; Nguyen, V.H.; Okamura, H.; Hamada, F.N. Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals. Genes Dev. 2018, 32, 140–155.
- Lin, Y.-J.; Seroude, L.; Benzer, S. Extended Life-Span and Stress Resistance in the Drosophila Mutant methuselah. Science 1998, 282, 943–946.
- Cvejic, S.; Zhu, Z.; Felice, S.J.; Berman, Y.; Huang, X.-Y. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat. Cell Biol. 2004, 6, 540–546.
- Harmar, A.J. Family-B G-protein-coupled receptors. Genome Biol. 2001, 2, 1–3013.
- Mezler, M.; Müller, T.; Raming, K. Cloning and functional expression of GABABreceptors from Drosophila. Eur. J. Neurosci. 2001, 13, 477–486.
- Adler, P.N.; Vinson, C.; Park, W.J.; Conover, S.; Klein, L. Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics 1990, 126, 401–416.
- I Strutt, D. Asymmetric Localization of Frizzled and the Establishment of Cell Polarity in the Drosophila Wing. Mol. Cell 2001, 7, 367–375.
- Povelones, M.; Nusse, R. The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling. EMBO J. 2005, 24, 3493–3503.
- Heuvel, M.V.D.; Ingham, P.W. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nat. Cell Biol. 1996, 382, 547–551.
- Fan, J.; Liu, Y.; Jia, J. Hh-induced Smoothened conformational switch is mediated by differential phosphorylation at its C-terminal tail in a dose- and position-dependent manner. Dev. Biol. 2012, 366, 172–184.
- Martin, B.L.; Kimelman, D. Wnt Signaling and the Evolution of Embryonic Posterior Development. Curr. Biol. 2009, 19, R215–R219.
- Villarreal, C.M.; Darakananda, K.; Wang, V.R.; Jayaprakash, P.M.; Suzuki, Y. Hedgehog signaling regulates imaginal cell differentiation in a basally branching holometabolous insect. Dev. Biol. 2015, 404, 125–135.
- Abrieux, A.; Duportets, L.; Debernard, S.; Gadenne, C.; Anton, S. The GPCR membrane receptor, DopEcR, mediates the actions of both dopamine and ecdysone to control sex pheromone perception in an insect. Front. Behav. Neurosci. 2014, 8, 312.
- Lark, A.; Kitamoto, T.; Martin, J.-R. Modulation of neuronal activity in the Drosophila mushroom body by DopEcR, a unique dual receptor for ecdysone and dopamine. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1864, 1578–1588.
- Lungchukiet, P.; Zhang, J.; Tobe, S.S.; Bendena, W.G. Quantification of allatostatin receptor mRNA levels in the cockroach, Diploptera punctata, using real-time PCR. J. Insect Physiol. 2008, 54, 981–987.
- Ida, T.; Takahashi, T.; Tominaga, H.; Sato, T.; Kume, K.; Ozaki, M.; Hiraguchi, T.; Maeda, T.; Shiotani, H.; Terajima, S.; et al. Identification of the novel bioactive peptides dRYamide-1 and dRYamide-2, ligands for a neuropeptide Y-like receptor in Drosophila. Biochem. Biophys. Res. Commun. 2011, 410, 872–877.
- Deng, X.; Yang, H.; He, X.; Liao, Y.; Zheng, C.; Zhou, Q.; Zhu, C.; Zhang, G.; Gao, J.; Zhou, N. Activation of Bombyx neuropeptide G protein-coupled receptor A4 via a Gαi-dependent signaling pathway by direct interaction with neuropeptide F from silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2014, 45, 77–88.
- Gross, A.D.; Temeyer, K.B.; Day, T.A.; de León, A.A.P.; Kimber, M.J.; Coats, J.R. Pharmacological characterization of a tyramine receptor from the southern cattle tick, Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol. 2015, 63, 47–53.
- Collin, C.; Hauser, F.; Krogh-Meyer, P.; Hansen, K.K.; De Valdivia, E.G.; Williamson, M.; Grimmelikhuijzen, C.J. Identification of the Drosophila and Tribolium receptors for the recently discovered insect RYamide neuropeptides. Biochem. Biophys. Res. Commun. 2011, 412, 578–583.
- Qi, Y.-X.; Xia, R.-Y.; Wu, Y.-S.; Stanley, D.; Huang, J.; Ye, G.-Y. Larvae of the small white butterfly, Pieris rapae, express a novel serotonin receptor. J. Neurochem. 2014, 131, 767–777.
- Vleugels, R.; Lenaerts, C.; Broeck, J.V.; Verlinden, H. Signalling properties and pharmacology of a 5-HT7-type serotonin receptor fromTribolium castaneum. Insect Mol. Biol. 2013, 23, 230–243.
- Cao, C.; Sun, L.; Du, H.; Moural, T.W.; Bai, H.; Liu, P.; Zhu, F. Physiological functions of a methuselah-like G protein coupled receptor in Lymantria dispar Linnaeus. Pestic. Biochem. Physiol. 2019, 160, 1–10.
- Pandey, A.; Khatoon, R.; Saini, S.; Vimal, D.; Patel, D.K.; Narayan, G.; Chowdhuri, D.K. Efficacy of methuselah gene mutation toward tolerance of dichlorvos exposure in Drosophila melanogaster. Free. Radic. Biol. Med. 2015, 83, 54–65.
- Tobin, A.B.; Butcher, A.J.; Kong, K.C. Location, location, location…site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol. Sci. 2008, 29, 413–420.
- Qi, Y.-X.; Xu, G.; Gu, G.-X.; Mao, F.; Ye, G.-Y.; Liu, W.; Huang, J. A new Drosophila octopamine receptor responds to serotonin. Insect Biochem. Mol. Biol. 2017, 90, 61–70.
- Van Hiel, M.B.; Vandersmissen, H.P.; Proost, P.; Broeck, J.V. Cloning, constitutive activity and expression profiling of two receptors related to relaxin receptors in Drosophila melanogaster. Peptides 2015, 68, 83–90.
- Yamanaka, N.; Yamamoto, S.; Žitňan, D.; Watanabe, K.; Kawada, T.; Satake, H.; Kaneko, Y.; Hiruma, K.; Tanaka, Y.; Shinoda, T.; et al. Neuropeptide Receptor Transcriptome Reveals Unidentified Neuroendocrine Pathways. PLoS ONE 2008, 3, e3048.
- Reim, T.; Balfanz, S.; Baumann, A.; Blenau, W.; Thamm, M.; Scheiner, R. AmTAR2: Functional characterization of a honeybee tyramine receptor stimulating adenylyl cyclase activity. Insect Biochem. Mol. Biol. 2017, 80, 91–100.
- Paluzzi, J.-P.; O’Donnell, M.J. Identification, spatial expression analysis and functional characterization of a pyrokinin-1 receptor in the Chagas’ disease vector, Rhodnius prolixus. Mol. Cell. Endocrinol. 2012, 363, 36–45.
- Hamoudi, Z.; Lange, A.B.; Orchard, I. Identification and Characterization of the Corazonin Receptor and Possible Physiological Roles of the Corazonin-Signaling Pathway in Rhodnius prolixus. Front. Neurosci. 2016, 10, 357.
- Paluzzi, J.-P.V.; Ebhatt, G.; Wang, C.-H.J.; Ezandawala, M.; Lange, A.B.; Eorchard, I. Identification, functional characterization, and pharmacological profile of a serotonin type-2b receptor in the medically important insect, Rhodnius prolixus. Front. Neurosci. 2015, 9, 175.
- Sha, K.; Conner, W.C.; Choi, D.Y.; Park, J.H. Characterization, expression, and evolutionary aspects of Corazonin neuropeptide and its receptor from the House Fly, Musca domestica (Diptera: Muscidae). Gene 2012, 497, 191–199.
- Verlinden, H.; Vleugels, R.; Verdonck, R.; Urlacher, E.; Broeck, J.V.; Mercer, A. Pharmacological and signalling properties of a D2-like dopamine receptor (Dop3) in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 56, 9–20.
- Stafflinger, E.; Hansen, K.K.; Hauser, F.; Schneider, M.; Cazzamali, G.; Williamson, M.; Grimmelikhuijzen, C.J.P. Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. Proc. Natl. Acad. Sci. USA 2008, 105, 3262–3267.
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Tobback, J.; Pflüger, H.-J.; Blenau, W.; Broeck, J.V. The cloning, phylogenetic relationship and distribution pattern of two new putative GPCR-type octopamine receptors in the desert locust (Schistocerca gregaria). J. Insect Physiol. 2010, 56, 868–875.
- Wang, S.; Wang, W.; Ma, Q.; Shen, Z.; Zhang, M.; Zhou, N.; Zhang, C. Elevenin signaling modulates body color through the tyrosine-mediated cuticle melanism pathway. FASEB J. 2019, 33, 9731–9741.
- Wu, S.-F.; Xu, G.; Qi, Y.-X.; Xia, R.-Y.; Huang, J.; Ye, G.-Y. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J. Neurochem. 2014, 129, 37–47.
- Xu, G.; Gu, G.-X.; Teng, Z.-W.; Wu, S.-F.; Huang, J.; Song, Q.-S.; Ye, G.-Y.; Fang, Q. Identification and expression profiles of neuropeptides and their G protein-coupled receptors in the rice stem borer Chilo suppressalis. Sci. Rep. 2016, 6, 28976.
- Zhang, F.; Wang, J.; Thakur, K.; Hu, F.; Zhang, J.-G.; Jiang, X.-F.; An, S.-H.; Jiang, H.; Jiang, L.; Wei, Z.-J. Isolation functional characterization of allatotropin receptor from the cotton bollworm, Helicoverpa armigera. Peptides 2019, 122, 169874.
- Pietrantonio, P.V.; Jagge, C.; Taneja-Bageshwar, S.; Nachman, R.J.; Barhoumi, R. The Mosquito Aedes aegypti (L.) leucokinin receptor is a multiligand receptor for the three Aedes kinins. Insect Mol. Biol. 2005, 14, 55–67.
- Esquivel, C.J.; Cassone, B.J.; Piermarini, P.M. Ade novotranscriptome of the Malpighian tubules in non-blood-fed and blood-fed Asian tiger Mosquitoes Aedes albopictus: Insights into diuresis, detoxification, and blood meal processing. PeerJ 2016, 4, e1784.
- Munoz, S.; Guerrero, F.D.; Kellogg, A.; Heekin, A.M.; Leung, M.-Y. Bioinformatic prediction of G protein-coupled receptor encoding sequences from the transcriptome of the foreleg, including the Haller’s organ, of the cattle tick, Rhipicephalus australis. PLoS ONE 2017, 12, e0172326.
- Verlinden, H.; Lismont, E.; Bil, M.; Urlacher, E.; Mercer, A.; Broeck, J.V.; Huybrechts, R. Characterisation of a functional allatotropin receptor in the bumblebee, Bombus terrestris (Hymenoptera, Apidae). Gen. Comp. Endocrinol. 2013, 193, 193–200.
- Kawakami, N.; Miyoshi, K.; Horio, S.; Fukui, H. β2-Adrenergic Receptor-Mediated Histamine H1 Receptor Down-Regulation: Another Possible Advantage of β2 Agonists in Asthmatic Therapy. J. Pharmacol. Sci. 2004, 94, 449–458.
- Drake, M.T.; Shenoy, S.K.; Lefkowitz, R.J. Trafficking of G Protein–Coupled Receptors. Circ. Res. 2006, 99, 570–582.
- Chen, J.-F.; Sonsalla, P.K.; Pedata, F.; Melani, A.; Domenici, M.R.; Popoli, P.; Geiger, J.; Lopes, L.V.; de Mendonça, A. Adenosine A2A receptors and brain injury: Broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog. Neurobiol. 2007, 83, 310–331.
- Duan, W.; Gui, L.; Zhou, Z.; Liu, Y.; Tian, H.; Chen, J.-F.; Zheng, J. Adenosine A2A receptor deficiency exacerbates white matter lesions and cognitive deficits induced by chronic cerebral hypoperfusion in mice. J. Neurol. Sci. 2009, 285, 39–45.
