The Central American locust (CAL), Schistocerca piceifrons piceifrons (Walker, 1870), is a transboundary pest that is distributed from Mexico to Panama. It is a true locust species characterized by density-dependent phase polyphenism. The ancient record of the CAL is found in the Popol Vuh, the Mayan sacred book, demonstrating how it has affected humans for millennia. In Mexico, the CAL has been declared a national threat to agriculture since 1824. Serious locust plagues occurred in 1882–1883 when swarms of 20 km2 in size invaded the Yucatán Peninsula and neighboring states in southern Mexico and, since then, management actions to suppress populations and economic damage have been implemented. A better understanding of the biology, ecology, and behavior of the CAL replaced a manual and mechanical collection of locust swarms, hopper bands, and egg pods with modern techniques such as the use of safer chemical products and environmentally friendly bioinsecticides. Presently, biomodels and GIS support the monitoring and forecasting of outbreaks.
- locust plagues
- ecology
- field monitoring
- outbreaks management
1. Background
The Central American locust (CAL), Schistocerca piceifrons piceifrons (Walker, 1870), is a menacing pest that has affected humanity for millennia [1]. It is believed that climate changes and recurrent locust plagues could have caused the decline of the Mayan civilization in Mesoamerica in the period 740–930 A.D. [1][2][3]. It is a transboundary migratory pest that is distributed throughout Mexico and Central America, affecting at least ten countries during outbreaks [4]. It is a true locust species characterized by density-dependent phase polyphenism [1][4][5][6][7][8]. At low density, individuals are solitary, avoid each other, and are harmless, and this phenotype is called the “solitary phase”; when environmental conditions promote population density increase, they transform into gregarious and conspicuous locusts that can form large migrating groups of nymphs (bands) or adults (swarms), and this phenotype is called the “gregarious phase”. Solitarious locusts differ from gregarious locusts not only in behavior, but also in color, morphology, and physiology [4][7][8][9]. Locust bands march in compact groups in the same direction and in a cohesive way, while swarms may contain millions of individuals that migrate long distances in a few days, causing severe damage to agriculture and livestock. The phenomenon that triggers polyphenism is not “unknown”, but it must be considered a very complex multifactorial process involving different mechanisms [10].
Throughout its distribution, permanent reproduction and gregarization areas for the CAL are located at 100 masl or below, which are characterized by optimal climatic conditions (27 °C average annual temperature, and at least 1000 mm annual precipitation) [4][5][9][11][12]. In modern Mexico, the first official report on the CAL plague dates back to 1882–1883 when swarms of 20 km2 in size invaded the Yucatán Peninsula and other states of southern Mexico [1][2][3][4][7]. Along with its history of massive reproduction and invasions, different management actions have been used to suppress infestations and economic damage [2][3][4][9]. A better understanding of the biology, ecology, and behavior of the CAL has gradually replaced the manual and mechanical collection of locust swarms, hopper bands, and egg pods performed during the 19th century with more appropriate management techniques [4][9][11][12][13][14][15][16]. At present, the use of biomodels and Geographic Information Systems Technology (GIS) support CAL monitoring and the forecasting of outbreaks [17][18][19]. During the most recent outbreak in 2018–2020, the National Service of Agri-food Health, Safety and Quality (SENASICA) introduced the potential use of unmanned aerial vehicles (UAVs) or drones for the monitoring of locust populations in addition to land monitoring [20][21]. Currently, several research lines elucidate on the evolution of phase polyphenism, the environmental factors that trigger gregarization and swarm formation, the bioactive compounds and nutritional contents of the CAL, and the improvement of an early warning system (EWS) [8][15][19][21][22][23][24][25][26]. Hopefully this work will provide novel information and knowledge that helps to improve the management and control strategies of the CAL.
2. Life History of the Central American Locust
It is well established that in continental Mexico and Central America, the CAL exhibit two generations per year [4][11][12][13][23][27][28][29]. Interestingly, only one generation per year is reported to occur in Socorro Island, Archipelago Revillagigedo by the Mexican Pacific Coast, 480 km southwest of Baja California, Mexico where a locust invasion was reported in 2006 [5][30]. In this review, we will refer to the life history of the CAL populations that occur in continental Mexico and Central America. In these regions, the second generation overwinters as sexually immature adults from November to April–May, undergoing a reproductive diapause. This physiological state is a response to the onset of the autumn-winter dry season that brings about a decrease in temperature and shorter days [4][9][23][27][28]. Adults remain sexually immature until the onset of the rainy season around mid-April. As climatic conditions improve, and when environmental conditions become suitable for reproduction, adults of the second generation become sexually mature and start mating and laying eggs from May through June. Sexually mature adults turn yellow and mate en masse on bare sandy-clay soils, where each female lays one to four egg pods at a depth of approximately 3 to 10 cm. Each egg pod may contain 40–75 eggs based on field observations [29]. These eggs will hatch from mid-May through June and give rise to the first generation. First-generation nymphs are present from May-June through July, and adults from mid-July to the end of September. The first generation develops very rapidly, given the favorable climatic conditions (27 °C average temperature, 50–80% relative humidity, and 13L: 11D period, as well as abundant and lush vegetation for feeding); it may take 55–60 days to progress from egg through to sexually mature adult. Mating and oviposition occur from mid-September through October. Second-generation nymphs may occur from mid-September through mid-December. By the end of November, 70–80% of the second-generation nymphs will have reached the adult stage and will remain sexually immature. There is an overlap between second-generation adults and nymphs of the first generation, and also between adults of the first generation and second-generation nymphs. The second generation has a longer duration, 155–180 days since the adult enters diapause in response to a drier season, lower temperature, and shorter day length that occurs in the region from November through April [4][7][9][11][12][17][20][23][27][28]. Under these unfavorable environmental conditions, the CAL may not resume development, though relative humidity occasionally may be high at 70–85% [4][9][27][28][29]. If the population density is high (>25–30 adults/m2) and environmental conditions become suitable, four to six weeks after reaching the adult stage, scattered populations may join to form swarms ready for migration. The first swarms in the region are observed and located from the second half of December onwards. The period, from December through April, is appropriate to undertake control measures since adult populations of the CAL remain sexually immature and in large aggregations (up to 5000 adults/m2) [9]. When the first rains begin in April–May, the adults then enter the process of sexual maturation and changes in their behavior and color continue to occur. The solitary young adult is brown with light-brown tones, and has a great capacity for flight over short distances of 15–20 m when disturbed; while the gregarious young adult is brown with dark brown tones and moves in a cohesive manner in large groups or swarms. As they reach sexual maturity, this coloration disappears and turns yellow, observed first in males and later in females, and is more conspicuous in gregarious than in solitary populations [4][9][27][28]. During this period, large numbers of locusts are observed flying against the wind, which helps to increase their body temperature and strengthen their flight muscles [4][9][11].
3. Habitat and Ecology
The CAL is a polyphagous species throughout its distribution area. It has been recorded to feed on up to 400 plant species, feeding on both cropped and grazing areas, including dry vegetation [4][9][27][28]. Feeding ecology of the CAL is not fully understood, but a recent study on the effect of plant volatiles and feeding preference of the CAL showed that the CAL in the Yucatán Peninsula was highly attracted to the odor of Pisonia aculeata L., which it uses as a refuge but not for feeding [22]. As for the feeding preference, the study revealed that the CAL showed marked feeding preference for two native plant species, Leucaena glauca Benth and Waltheria americana L. [22]. However, it is not clear if this observation can be applied to other regions where the CAL is present. In Mexico and Central America, the breeding areas for the CAL are cultivated and natural grasslands that have replaced the low and thorny forests. For instance, in the coastal plain of the Gulf of Mexico and the Yucatán Peninsula, 80% of the areas of reproduction and gregarization occur in these agroecosystems [19]. The habitat of solitarious populations is characterized by grassland and vegetation patches that do not exceed 2.5 m in height [4]. Its distribution depends upon the vegetation pattern and, if the vegetation is homogeneous, locust populations may remain scattered. However, anthropogenic activities, such as burning or abandonment of cultivated areas, and environmental factors (convergent winds, droughts, flooding, and food shortage) may lead to gregarization and swarm formation [4][19][28][29]. In recent years, changes in land use through the conversion of forested areas into agricultural areas have aggravated the locust problem, since the area suitable for reproduction and gregarization has increased. For example, in the Yucatán Peninsula, which represents the most important breeding area in Mexico, the agricultural area increased from 394,236 ha in 1981 to 579,643 ha in 2014; in a 30 year period, 47% of the forested area was converted to livestock activities [31]. Recent studies found that the burning of grassland and agricultural areas that are ideal for feeding, reproduction, and oviposition of the CAL are associated with the emissions of methane (CH4) and carbon dioxide (CO2), and these greenhouse gases increase soil temperature up to 3°C which favors locust breeding and gregarization, as occurs in northern Mexico, north of the Huasteca Potosina and southern Tamaulipas, where locust populations have increased [19][32].
4. Permanent Breeding Areas
Solitarious populations of the CAL reproduce and develop in permanent breeding areas located in the lowlands that provide suitable climatic conditions for reproduction, uncovered land or with little vegetation cover, and close to cultivated areas. The invasion area extends south of the Tropic of Cancer (23°26′), at no more than 2000 masl and 700–2500 mm annual precipitation [28][33][34]. The onset of the spring rains is critical to induce sexual maturation, mating, and oviposition of second-generation adults [4][9][16][28][33][34]. Four permanent breeding areas have been recognized [4][11][13] (Figure 1): (1) the Yucatán Peninsula in Mexico; (2) the Valley of Rio Aguan in Northern Honduras; (3) the Gulf of Fonseca in El Salvador, Honduras, and Nicaragua; and (4) the Province of Guanacaste in Costa Rica. Recently, four additional breeding areas have been identified in Mexico [34][35] (Figure 1): (5) the State of Veracruz, municipalities of Medellín, Boca del Río, Alvarado, Tlalixcoyan, and Tierra Blanca, which is second in importance after the Yucatán Peninsula; (6) Chahuites-Tepanatepec, located on the limits of Oaxaca and Chiapas, where the CAL becomes gregarious, causing invasions into the Isthmus of Tehuantepec and other localities in the state of Oaxaca; (7) the border of San Luis Potosí-Tamaulipas states, in the Valleys of the Sierra Nahola, the smallest of the four additional permanent breeding areas, which currently has given rise to serious invasions into the Huasteca region (East of San Luis Potosi, South Tamaulipas, and North of Veracruz); and (8) Tabasco, located on the banks of the Usumacinta River on the border with Guatemala, where it is considered that environmental conditions can lead to the development of CAL populations.
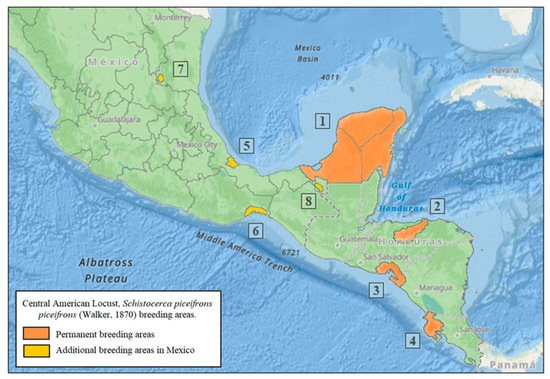
Figure 1. Central American locust, Schistocerca piceifrons piceifrons (Walker, 1870), permanent breeding areas: (1) Yucatán Península, México, (2) Valley of Rio Aguan, Northern Honduras, (3) Gulf of Fonseca in El Salvador, Honduras, and Nicaragua, (4) Province of Guanacaste, Costa Rica, (5) State of Veracruz, México, (6) Chahuites-Tepanatepec, on the limits of Oaxaca and Chiapas, Mexico, (7) Border of San Luis Potosí-Tamaulipas states, México, and (8) Tabasco, on the banks of the Usumacinta River, border with Guatemala.
Seasonal migration between habitats does not seem to be part of the life-cycle of the solitarious phase, although conclusive studies have not been made. Plagues occur when swarms form and migrate outside of the permanent breeding sites. The ecological factors that cause outbreaks are poorly understood; nonetheless, the extent of a suitable habitat created by anthropogenic activities plays a major role [4][9][11][12][13][16][19]. Several works have associated CAL outbreaks with the event of El Niño Southern Oscillation (ENSO) (a drought period followed by abundant rain) and La Niña [19][36]. Recent studies found that solitarious populations of the CAL in the Yucatán Peninsula are high in the rainy season and correlated with the abundance of the grass Panicum maximum Jacq. and precipitation [37]. Indeed, precipitation is one of the most influential factors in determining locust activities, such as mating and oviposition periods. Humidity is required in the soil to ensure the hatching of the eggs and to satisfy the water requirements of nymphal development [4][9][11][12][13][16][17][23][38]. Regarding the CAL plagues and invasions, the coastal lowlands of Mexico have been invaded as far north as the Tropic of Cancer by swarms originating in the Yucatán Peninsula or the Pacific Coast of Guatemala [13][39]. In 1923, a CAL plague invaded the state of Veracruz [13][40]. Swarms occupied the hot coastal plain (Tierra Caliente) where they bred. However, adults moved to the west, up the valleys towards the cooler highlands where they bred, but hoppers perished because of the cool weather. Prevailing winds and the occurrence of rain all contributed to determining the direction of swarm movements. It has been shown that, in the Yucatán Peninsula, the migration direction of the first-generation swarms is northeast and of the second-generation swarms is southwest [37].
5. Band and Swarm Formation
Solitarious nymphs of the CAL are green or tan in color, avoid encounters with other individuals, and cause no economic damage. As the local population density increases due to favorable environmental conditions, they become gregarious and attracted to each other. Soon they develop a pink to orange background color with intense black patterns (Figure 2) [4][5][6][9][41].
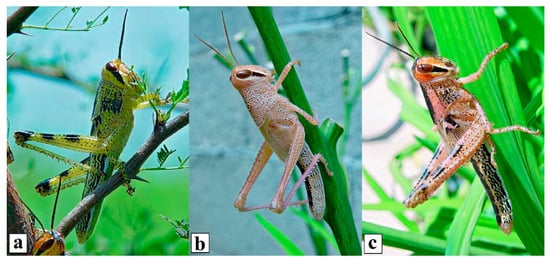
Figure 2. Central American locust, Schistocerca piceifrons piceifrons (Walker, 1870), solitarious (a,b) and gregarious (c) nymphs showing different color patterns.
Prolonged high-density conditions may result in an exponential population growth, which can lead to the formation of bands consisting of millions of individuals that march in the same direction in a cohesive manner [4][5][9][11][12][13][41]. Displacement of locust bands depends upon developmental stages and environmental factors; the first and second nymphal instars of the gregarious phase may remain on the host plant for a few days, but older nymphal instars (third-sixth) form bands and may displace 15–50 m per day [4][11][28]. It is reported that the black pigmentation allows the insects to be more efficient at absorbing solar radiation, raising their body temperature and metabolic activity; this is important during the winter (diapause stage) [4][11]. Other adaptive significance of the black patterns, such as aposematic coloration, which was shown in the desert locust [42], has not yet been demonstrated in the CAL. Gregarization is also associated with drastic changes in the insect’s habitat or unusual climatic changes, such as convergent air currents. If the population density is high (>5 nymphs/m2) and harvesting, burning of crop remnants, or soil preparation activities are performed, the nymphs gather and form compact groups (bands) on patches of vegetation nearby the cultivated land [9][29][41]. Upon reaching the adult stage, these small groups, which can vary in size from a few m2 to several hectares, join with others and form swarms. This behavior may be observed at the end of crop harvest in the spring-summer cycle. At this stage, there is an opportunity to undertake control measures since it is easier to deal with small areas already located than large areas of land when the population is dispersed [4][9][16][27][28][29]. Further research is necessary to understand the gregarization mechanism and factors involved in the displacement of locust bands and swarms.
6. Phase Polyphenism, Gregarization, and Migration
The CAL is a true locust species that shows an extreme form of density-dependent phenotypic plasticity [4][5][6][9][16][27][28][29][41]. While the proximate mechanisms of phase transformation in the CAL are less understood than its congener S. gregaria, recent laboratory research has shown that the rearing density has a dramatic impact in resulting phenotypes including color, morphology, and behavior. Similar to other locust species, the solitarious CAL avoid each other, are inconspicuous, and harmless, while the gregarious CAL are attracted to each other, are conspicuously colored, and form large aggregations of nymphs (bands; Figure 3) or adults (swarms; Figure 4) that migrate long distances causing severe damage to agriculture and humankind [4][5][6][11][12][13][27][28][29][41].

Figure 3. Central American locust, Schistocerca piceifrons piceifrons (Walker, 1870), 1st (a) and 6th (b) instar bands.

Figure 4. Central American locust, Schistocerca piceifrons piceifrons (Walker, 1870), swarms.
It is unclear at this point what the relative importance of tactile, olfactory, and visual stimuli is in detecting changes in density in the CAL, but the preliminary laboratory data seem to suggest that the proximate mechanisms of phase transformation may be slightly different from what is known in the desert locust [43]. For example, the relative rates of gregarization and solitarization are different from the desert locust (Foquet et al., unpublished). This is not surprising given the fact that the CAL has evolved phase polyphenism independently from the desert locust [44]. The molecular basis of phase polyphenism in the CAL is currently being studied using transcriptomics and RNA interference. Phenotypic plasticity is often considered to be an adaptation to heterogeneous environment [45], but whether the phase polyphenism in the CAL is adaptive or not has not yet been formally studied. Currently, it is not known whether the conspicuous coloration of the gregarious nymphs is aposematic, because it is frequently observed in the field that vertebrate predators readily consume gregarious nymphs. However, it is possible that these insects may prefer to feed on toxin plants at the early stage of gregarization, which will provide a certain level of chemical protection. This is an untested hypothesis, but a previous study on the desert locust demonstrated that at the early stage of gregarization, the nymphs would preferentially feed on toxic plants, which makes their yellow and black coloration aposematic [42]. Other adaptive features of the nymphal coloration, such as thermoregulation or heightened immunity [45], have not yet been studied in the CAL [Song, pers. comm.]. Likewise, the adaptive nature of cryptic coloration and solitary behaviors associated with the solitarious phase have not been tested, but it would be quite easy to imagine that they would be adaptive.
The solitarious nymphs in the field setting are often green with small black dots and markings on the pronotum, abdomen, and hind legs, while the gregarious nymphs have a pink or peach background color with an extensive black pattern on the head, pronotum, abdomen, pads, and legs (Figure 2 and Figure 3) [4][5]. Newly fledged adults are brown to pinkish in color, with abundant light black marks on the tegmina. Sexually immature adults in the solitarious phase are brown with light-brown tones and have a great capacity for flight. Sexually immature adults in the gregarious phase exhibit a darker pink color with darker marks on the tegmina and turn bright yellow on sexual maturity, the latter being observed first in males and later in females (Figure 5) [4][5][9][11][12][27][28][29][41].

Figure 5. Central American locust, Schistocerca piceifrons piceifrons (Walker, 1870), sexually immature adults in the solitarious (a,b) and gregarious phase (c); sexually mature adult in gregarious phase showing bright yellow color (d).
Sexual maturity is closely related to precipitation and relative humidity, with second-generation adults beginning this process with the onset of rains in April–May; during the next three to four months, thousands of yellow adult couples will mate and lay eggs simultaneously in bare, rich-clay soils [4][5][9][11][12][27][28][29][41]. Under laboratory conditions, a similar pattern has been observed, with small differences [4][9][11][12][27][28][29]: rearing density affected the hopper coloration greatly. Those reared in crowds invariably had a heavy black pattern on a pink or peach ground color; those reared in isolation had little or no black pattern and the ground color was green, brown, pink, or straw. The type of food also affected the coloration of the isolated hoppers, but had no effect on the crowded specimens. The color of fledgling adults was not correlated with nymphal coloration; all had a rather somber, brown appearance. Sexually mature males kept in crowds became predominantly yellow, but crowded females developed only a little yellow coloration, and isolated adults developed none at all upon maturity. In captivity, density also affected the length of the pre-oviposition period, which averaged 36 days from fledging to the production of the first egg pod by females kept in crowds and 61 days for those reared in isolation. Crowded adult females averaged six egg pods each, with 70 eggs per pod; while isolated females averaged eight pods, with 62 eggs per pod. Egg pods were produced at 6- to 7-day intervals, regardless of density. The incubation period was 18–19 days at 32°C and 25 days at 28°C. It is documented that adults of the CAL strengthen their flight muscles four to six weeks after reaching the adult stage, and if population density is high (>10–15/m2) and environmental factors suitable, scattered populations join forming swarms that start migrating [4][9][16]. Swarm migration occurs mainly during the day, but occasionally swarms have been seen flying at night when the temperature is high (25°C ± 2°C) and on light moon nights. Second-generation swarms are observed and located in the region from the second half of December onwards [4][9][27][28][29]. During this stage, it is advisable to undertake all possible control actions since adults have not mated or oviposited and remain at very high population densities (up to 5000 adults/m2) in large aggregations (Figure 4).
Large numbers of locusts are frequently observed flying against the wind to increase their body temperature [4][9][28]. In regard to environmental factors, such as temperature, precipitation, day length, distribution of vegetation patterns, and land use, it is well established that all play a major role in influencing and inducing gregarization and phase transformation [4][9][13][16][18][19][23][27][28]. Recent work showed that in the Yucatán peninsula, which represents the most important breeding area in Mexico, environmental factors associated with the population density of the CAL are, in order of importance, the relative species density of vegetation, the isotherm and isohyets, maximum precipitation, temperature, and land use; the population density of the CAL is influenced primarily by the abundance of the grass P. maximum and precipitation [23].
7. Central American Locust Outbreaks
The CAL, like its congeners, has caused fear and destruction throughout its distribution area for centuries. In Mexico, the most ancient records come from pre-Hispanic times [1][2][3][14] and the locust swarms are believed to be one of the causes that led to the decline of the Maya-Quiché cultures in the Yucatán Peninsula and the Toltec in the Anahuac Valley. The Mayan sacred book, the Popol-Vuh, mentions that the ancient people migrated out of hunger brought about by the agricultural losses inflicted by the locust plagues. During the colonial time, written records on locust plagues date back to the seventeenth century, when the first invasion in the territory currently occupied by the Yucatán Peninsula, Tabasco, Chiapas, Oaxaca, and Veracruz, was reported [38][39][46][47]. In Mexico, the CAL was declared a national pest in 1824 and provisions were established to fight off bands and swarms [47]. In 1924–1926, there was a great invasion and, since then, the presence of locust plagues has occurred without interruption in one or another part of its distribution area, especially in the Yucatán Peninsula and Central America [4][9][14][19][21]. The National Plant Protection Service in Mexico created a permanent locust control campaign in 1949 [48][49]. The OIRSA (Organismo Internacional Regional de Sanidad Agropecuaria) was created in 1947 as the CICLA (Comité Internacional Contra la Langosta), and in 1955 the CAL was declared a transboundary pest that required the coordinated efforts of Central American countries to deal with it. At present, the OIRSA represents the Central American anti-locust organization and continues to be in charge of coordinating the regional control efforts, funding anti-CAL campaigns, training, and providing information on the current locust situation [21][49][50]. From 2016 to date, there have been significant population increases of the CAL in the OIRSA Region. For example, in Nicaragua in 2016, locust swarms were controlled by the Institute for Agricultural Protection and Health (IPSA). There were outbreaks in the Yucatán Peninsula, Mexico (2014–2020), El Salvador (2016 and 2018) and, at present, after a long recession period, outbreaks were registered in Belize. Significant populations of the CAL have been reported in Guatemala, suggesting that there are factors that favor the development of this pest. On 2 July 2020, OIRSA issued a regional alert for the prevention of the CAL swarms [50].
8. Central American Locust Management Strategies
Considerable progress has been made on the management of the CAL during recession and outbreak periods [9][16][17][18][19][36][51][52][53][54][55][56][57][58][59][60][61][62]. Management techniques have evolved from the manual and mechanical collection of egg pods, hopper bands, and adult swarms [1][2][3][4][14] to the use of prediction models that aim to understand how population dynamics of the CAL relate to environmental factors and local conditions. For instance, a study [36] on the relationship between sea surface temperatures, the El Niño Southern Oscillation (ENSO), and the potential of the CAL plagues found a 72% coincidence between years of massive CAL attacks and ENSO years in the North Pacific of Costa Rica; an increase of environmental temperature and irregular distribution of precipitation, as occurs during the ENSO years, may result in vigorous copulation and oviposition in the CAL. A population increase is the first step for phase change and eventual plagues. A subsequent work assessed the risk of next-generation nymph-populations, considering an initial adult population and each instar mortality rate [51]. In another study, a Thermal Time Locust Development Clock (TTLDC) was built considering average values of temperature, day length, diapause cool hours, and calendar dates; this model helped to predict the CAL timing of phenological stages. This model implies a starting date (Bio-fix) established experimentally after quality data on population dynamics and the life cycle of the CAL. When the model was compared with field observations, the differences were relatively small. The reliability of predictions depends upon the accuracy of the starting date and deviations in weather conditions from the average year [17]. More recently, an early warning system (EWS) for monitoring and management of the CAL habitat in permanent breeding areas in the Huasteca region (Mexican Coastal Plain) and the Yucatán Peninsula was implemented by SENASICA-UASLP (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Dirección General de Sanidad Vegetal-Universidad Autónoma de San Luis Potosí). This EWS applies multi-criteria models and NOAA-AVHRR satellite images [15][19][32]. The model is based on two variables of the meteorological mesoscale: the presence of the ENSO and drought monitoring; it also takes the CAL behavior into account. Accordingly, the process of band or swarm formation begins when solitarious populations face environmentally unfavorable conditions (grass or crop remnant burning, convergent winds, droughts, or food shortage) and converge in places where vegetation is available. The increase in population density of individuals per unit area leads the insect to gregarize and prepare to migrate in search of food and oviposition sites. Hence, climatic effects are among the fundamental cause of behavioral change, and the host distribution pattern reflects spatially the areas of gregarization and oviposition (greater stress), and the areas of migration in search of food (higher biomass production in agricultural areas). Currently, this EWS proposes that not only the ENSO, but also the La Niña, affects locust breeding and gregarization. Furthermore, there is evidence that as a consequence of the increase of greenhouse gas emissions such as CH4 and CO2 in gregarious areas, in the last four years (2017–2020), the drought monitor showed an increase of more than 3 °C in soil temperature in areas proximate to the Gulf of Mexico. These greenhouse gas emissions (CH4 and CO2) are produced by anthropogenic livestock activity, which creates more favorable environmental conditions for the reproduction of the CAL [32]. An additional dynamic simulation model [18] showed that the growth and development of the CAL in the Yucatán Peninsula was associated with daily rainfall, temperature, and physical soil properties, such as texture and depth. In regard to the growth of non-cultivated grass in breeding zones and oviposition rates, the model estimated both variables as a function of soil moisture. The latest work [23] focused on a deeper analysis of the environmental factors that affect population dynamics of the CAL and it was found that plant species richness (PSR) and relative species density (RSD) in the Yucatán Peninsula were higher during the rainy season than in the dry season, with RSD the most important variable associated with locust density, followed by isotherm and isohyets, maximum precipitation and temperature, and land use. Locust density was positively correlated with the abundance of the grass P. maximum. Therefore, surveys for early detection and control of the CAL on the Yucatán Peninsula may focus on areas with the grass P. maximum to predict risk areas and target survey efforts. In the most recent outbreak (2014–2020) in the Yucatán Peninsula, the possibility of using drones or unmanned aerial vehicles (UAVs) for locust surveying was introduced [49][50][52]. Hopefully, the Mexican National Plant Protection Service and the OIRSA will have trained technicians in the near future to incorporate this tool in the surveying of locust populations and into the CAL campaigns. All these efforts have provided novel information and additional techniques for the CAL surveying and management. However, future research integrating multiple disciplines is needed to obtain finer data on the ecological factors and their correlation with the population dynamics of the CAL. Moreover, all information generated must be incorporated into the CAL management strategy since, at the moment, the use of the available techniques and tools is limited.
9. Control Measures
Control campaigns in Mexico and Central America aim to prevent swarm formation or migration by undertaking timely control of locust bands and swarms through the continuous surveying and monitoring of breeding areas in the Mexican Coastal Plain, southern Mexico, and six Central American countries [4][7][11][12][13][19][20][21][27][34][35][47]. This preventive control strategy is occasionally halted due to a lack of economic resources, shortage of personnel, or climatic conditions. In addition, deforestation, grassland burning, and livestock activity have intensified in recent years, increasing the area with suitable conditions for reproduction and growth of the CAL [22][23][41][49]. Up to the present, chemical control is perhaps the main alternative during outbreaks. Previous to 1998, most control operations against the CAL were carried out using methyl parathion. However, beginning in 1999–2000, chemical control was diversified with the validation and introduction of additional products such as phenylpyrazole, pyrethroids, insect growth regulators, and, above all, bioinsecticides [16][28][50][52][53][54][55][56][57][58][59][60][61][62]. From 2000–2010, a successful biological control program was established. This program employed searching for local strains of entomopathogenic fungi, screening for the most pathogenic and virulent strains, development and formulation of a biological insecticide supported in laboratory and field trials, and the transference of technology to the National Plant Protection Service to produce the bioinsecticide [16][28][53][54][55][56][57][58][59][60][61][62]. Metarhizium anisopliae acridum, a native isolate, is produced at present by the National Plant Protection Service in two state-run laboratories in Guanajuato and Yucatán, and a privately-owned business in Puebla [16][28][53][54][55][56][57][58][59][60][61][62]. Field trials in the Huasteca region and the Yucatán Peninsula (4 × 1012 conidia per hectare) in a mineral oil formulation provided up to 90% mortality in swarms following applications of the pathogen. At present, this biological insecticide is used to suppress locust bands to prevent swarm formation and, during the winter season, it is used to suppress locust swarms over grassland areas. In 2010, the fungus was applied to over 4000 ha of locust at a cost of approximately USD 10 per hectare [60]. Neem extracts (Azadirachta indica Juss) are recommended by OIRSA as a viable alternative to suppress nymph and adult populations of the CAL that invade forest or urban areas. It is reported that at a dose of 1 L/ha applied in 1–2 L of vegetable oil, neem extracts cause 67% mortality after 48 h and 100% after 7 days [34][50]. Safer chemical products, bioinsecticides, and neem extracts, as well as the introduction of new technologies, have been integrated into the locust preventive management strategy, making the Integrated Pest Management approach, adopted in 2005, possible [61][62].
This entry is adapted from the peer-reviewed paper 10.3390/agronomy11061024
References
- Flores-Granados, F. Las Plagas de Langosta en el Área Maya: Ambiente e Historia de Una Calamidad en la Época Prehispánica. Península 2011, 6, 27–46. Available online: (accessed on 10 January 2021).
- García-Quintanilla, A. La Langosta, los Mayas y el Colonialismo en Yucatán, México, 1883. Relaciones 2012, 129, 215–249. Available online: (accessed on 10 January 2021).
- García-Quintanilla, A. Saak’ y el Retorno del Fin del Mundo: La Plaga de Langosta en las Profecías del Katun 13 Ahau. Ancient Mesoamerica 2005, 16, 327–344. Available online: (accessed on 10 January 2021).
- Barrientos-Lozano, L.; Astacio-Cabrera, O.; Poot-Pech, M.; Cruz-Martínez, O. Manual Técnico Sobre la Langosta Voladora (Schistocerca Piceifrons Walker 1870) y Otros Acridoideos de Centro América y Sureste de México, 1st ed.; Fao-Oirsa: San Salvador, El Salvador, 1992; pp. 1–162.
- Song, H.; Weissman, D.B.; Barrientos-Lozano, L.; Cano-Santana, Z. The Locust Island. Am. Entomol. 2009, 52, 168–181.
- Song, H. Density-dependent phase polyphenism in nonmodel locusts: A minireview. Psyche 2011.
- Senasica-DGSV. Langosta Centroamericana [Schistocerca piceifrons piceifrons (Walker, 1870)] (Orthoptera: Acrididae), Ficha Técnica; National Service of Agrifood Health, Safety and Quality-General Directorate of Plant Health-National Center for Phytosanitary Reference-Phytosanitary Specialist Group: Ciudad de México, Mexico, 2016; pp. 1–18. Available online: (accessed on 11 January 2021).
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Berry, K.; Buhl, J.; de Keyser, R.; Foquet, B.; Hadrich, J.C.; Matheson, T.; et al. From molecules to management: Mechanisms and consequences of locust phase polyphenism. Adv. Insect Phys. 2017, 53, 169–285.
- Ávila-Valdez, J.; Barrientos-Lozano, L.; García-Salazar, P. Aspectos Bio-Ecológicos de la Langosta Centroamericana en el Sur de Tamaulipas. In Simposio Control Biológico y Manejo de la Langosta Centroamericana Schistocerca piceifrons piceifrons (Walker); Instituto Tecnológico de Cuidad Victoria: Ciudad Victoria, Tamaulipas, Mexico, 2007; pp. 1–21.
- Pflüger, H.J.; Bräunig, P. One hundred years of phase polymorphism research in locusts. J. Comp. Physiol. 2021, 207, 321–326.
- Astacio-Cabrera, O. La Langosta Voladora o Chapulín Schistocerca piceifrons piceifrons (Walker) en Centroamérica, 1st ed.; Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA): San Salvador, El Salvador, 1990; pp. 1–42.
- Hunter-Jones, P. Life history of the Central American Locust, Schistocerca sp. (Orthoptera: Acrididae), in the laboratory. Ann. Entomol. Soc. Am. 1967, 60, 468–477.
- Harvey, A.W. Schistocerca piceifrons (Walker) (Orthoptera: Acrididae), the swarming locust of tropical America: A review. Bull. Entomol. Res. 1983, 73, 171–184.
- Ortiz-Yam, I.; Zuleta, M.C. Asuntos de vecinos: Langosta, defensa agrícola y la construcción de la sanidad vegetal en México y Centroamérica, siglo XX. Historia Méxicana 2019, 70, 313–373.
- Conteras-Servín, C.; Magaña-Ortíz, C. Ficha Técnica de la Langosta Centroamericana, Schistocerca piceifrons piceifrons (Walker). In La Plaga de la Langosta (Schistocerca piceifrons piceifrons Walker) Una Visión Multidisciplinaria Desde la Perspectiva del Desastre Fitosanitario en México, 1st ed.; Galindo Mendoza, G., Contreras Servín, C., Ibarra Zapata, E., Eds.; Universidad Autónoma de San Luis Potosí: San Luis Potosí, Mexico, 2013; Volume 1, pp. 17–36.
- Barrientos-Lozano, L. Taller Sobre Control. In Biológico y Manejo de Langosta Centroamericana (Schistocerca Piceifrons Piceifrons, Walker), 1st ed.; Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Instituto Tecnológico de Ciudad Victoria: Ciudad Victoria, Tamaulipas, Mexico, 2007; pp. 1–197.
- Rodríguez-Absi, J.; Almaguer-Sierra, P.; Barrientos-Lozano, L.; Rodríguez-Fuentes, H. Thermal time clock for estimating phenological development of Schistocerca piceifrons piceifrons Walker (Orthoptera: Acrididae) in northeastern Mexico. J. Orthoptera Res. 2009, 18, 65–73.
- Hernández-Zul, M.I.; Quijano-Carranza, J.A.; Yañez-López, R.; Ocampo-Velazquez, R.V.; Torres-Pacheco, I.; Guevara-Gonzalez, R.G.; Castro-Ramírez, E. Dynamic simulation model of Central American Locust Schistocerca piceifrons (Orthoptera: Acrididae). Fla. Entomol. 2013, 96, 1274–1283.
- Galindo-Mendoza, M.G.; Ibarra-Zapata, E.; Hipólito-Cruz, G.; Aldama-Aguilera, C. Sistema de Alerta Temprana Para el Control de la Langosta Centroamericana (Schistocerca piceifrons piceifrons (Walker) en la Península De Yucatán Aplicando Modelos Multicriteria e Imágenes de Satélite NOAA-AVHRR. In La Plaga de la Langosta, Schistocerca piceifrons piceifrons (Walker), una Visión Multidisciplinaria Desde la Perspectiva del Desastre Fitosanitario en México, 1st ed.; Universidad Autónoma de San Luis Potosí: San Luis Potosí, Mexico, 2013; pp. 173–215.
- Senasica-DGSV. Manual Operativo de la Campaña Contra la Langosta Centroamericana [Schistocerca piceifrons piceifrons (Walker, 1870)] (Orthoptera: Acrididae), 1st ed.; Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria–Dirección General de Sanidad Vegetal: Ciudad de México, Mexico, 2019; pp. 1–80. Available online: (accessed on 15 January 2021).
- Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA). Plan de Acción de Manejo de la Langosta Centroamericana, 1st ed.; Secretaría General del Sistema de la Integración Centroamericana: San Salvador, El Salvador, 2020; pp. 1–80. Available online: (accessed on 15 January 2021).
- Poot-Pech, M.A.; Ruiz-Sánchez, E.; Ballina-Gómez, H.S.; Gamboa-Angulo, M.M.; Reyes-Ramírez, A. Olfactory response and host plant feeding of the Central American Locust Schistocerca piceifrons piceifrons Walker, to common plants in a gregarious zone. Neotrop. Entomol. 2016, 45, 382–388.
- Poot-Pech, M.A.; Ruiz-Sánchez, E.; Gamboa-Angulo, M.; Ballina-Gómez, H.S.; Reyes-Ramírez, A. Population fluctuation of Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) in the Yucatán peninsula and its relation with the environmental conditions. Rev. Biol. Trop. 2018, 66, 403–414.
- Pérez-Ramírez, R.; Torres-Castillo, J.A.; Barrientos-Lozano, L.; Almaguer-Sierra, P.; Torres-Acosta, R.I. Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) as a source of compounds of biotechnological and nutritional interest. J. Insect Sci. 2019, 19, 1–9.
- Viesca-González, F.C.; Romero-Contreras, A.T. La entomofagia en México. Algunos aspectos culturales. El Periplo Sustentable 2009, 16, 57–83. Available online: (accessed on 16 January 2021).
- Garza-Sánchez, J. Evaluación del Potencial Alimenticio de la Langosta Centroamericana, Schistocerca piceifrons piceifrons, (Walker, 1870), (Orthoptera: Acrididae). Master’s Thesis, Tecnológico Nacional de México-Instituto Tecnológico de Cd. Victoria, Victoria, Tamaulipas, Mexico, 21 October 2020.
- Barrientos-Lozano, L. Population Dynamics, Biology and Ecology of the Central American Locust (Schistocerca piceifrons piceifrons, Walker) in Southern Mexico. In Proceedings of the Metaleptea—Eight International Meeting of the Orthopterists’ Society, Montpellier, France, 19–22 August 2001; International Conference of Orthopteroid Insects. p. 75.
- Barrientos-Lozano, L. Ecología, Manejo y Control de la Langosta Voladora, Schistocerca piceifrons piceifrons (Walker). In Proceedings of the Primer Curso Internacional: Ecología, Manejo y Control de la Langosta Voladora, Schistocerca piceifrons piceifrons (Walker), Altamira, Tamaulipas, México, 5–7 November 2001; Barrientos-Lozano, L., Ed.; Dinámica Impresa S.A.: Cuidad Victoria, Tamaulipas, Mexico, 2001; pp. 1–232.
- Ávila-Valdez, J.; Barrientos-Lozano, L.; García-Salazar, P. Biología y comportamiento de la langosta centroamericana, Schistocerca piceifrons piceifrons (Walker, 1870), en el Sur de Tamaulipas. In Proceedings of the 2do Curso Internacional: Manejo Integrado de la Langosta Centroamericana y Acridoideos Plaga en América Latina, Cuidad Victoria, Tamaulipas, Mexico, 27–29 June 2005; Barrientos-Lozano, L., Almaguer-Sierra, P., Eds.; Dinámica Impresa S.A.: Cuidad Victoria, Tamaulipas, Mexico, 2005; pp. 31–35.
- Cano-Santana, Z. Ecología e Historia Natural de Schistocerca americana socorro y S. piceifrons piceifrons en Isla Socorro; Informe Final Proyecto BS007; Universidad Nacional Autónoma de México: Ciudad de México, México, 2006; pp. 1–138. Available online: (accessed on 1 February 2021).
- Poot-Pech, M.A. La Langosta Voladora Schistocerca piceifrons (Orthoptera: Acrididae), Hacia un Manejo Sustentable. In El Patrimonio, su Importancia y Conservación: Conociendo el Patrimonio, 1st ed.; TECCIS: Ciudad de Campeche, Campeche, Mexico, 2016; pp. 58–66. Available online: (accessed on 4 February 2021).
- Galindo-Mendoza, M.G.; Contreras Servín, C. Alerta Climática-Fitosanitaria Para: Langosta Centroamericana, Schistocerca piceifrons piceifrons (Walker, 1870), Zona Huasteca, San Luis Potosí. In Sistemas de Alerta Temprana en Actividades Agropecuarias con Tecnología Espacial; SENASICA-UASL: San Luis Potosí, Mexico, 2021.
- Barrientos-Lozano, L. Ortópteros Plaga de México y Centro América: Guía de Campo, 1st ed.; Instituto Tecnológico de Cd. Victoria, COSNET, SEP-CONACYT: Cuidad Victoria, Tamaulipas, Mexico, 2003; pp. 1–114.
- Garza-Urbina, E. La langosta Schistocerca piceifrons piceifrons y su Manejo en la Planicie Huasteca; Folleto Técnico No. 12; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), CIRNE: San Luís Potosí, Mexico, 2005; pp. 1–23. Available online: (accessed on 4 February 2021).
- Senasica-DGSV. Memoria del Curso Regional Sobre la Langosta (Schistocerca piceifrons piceifrons); Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Dirección General de Sanidad Vegetal, CIRNE, INIFAP, SAGARPA: San Luis Potosí, Mexico, 2003; pp. 1–93.
- Retana, B.J.A. Relación Entre Algunos Aspectos Climatológicos y el Desarrollo de la Langosta Centroamericana Schistocerca piceifrons piceifrons en el Pacífico Norte de Costa Rica Durante la Fase Cálida del Fenómeno El Niño-Oscilación Sur (ENOS). Tóp. Meteorol. Oceanogr. 2000, 7, 73–87. Available online: (accessed on 4 February 2021).
- Poot-Pech, M.A.; Ruiz-Sánchez, E.; Ballina-Gómez, H. Indicators Plants in Solitary Phase and Migration Behavior of Schistocerca Piceifrons in Yucatán, México. Metaleptea 2017, 37, 21.
- Rainey, R. Meteorology and the Migration of Desert Locusts: Applications of Synoptic Meteorology in Locust Control; Technical Note No. 54; World Meteorological Organization (WMO): Geneva, Switzerland, 1988; pp. 1–115. Available online: (accessed on 4 February 2021).
- Márquez-Delgado, A. La Lucha Contra la Langosta en México, 1st ed.; Colegio de Ingenieros Agrónomos: Ciudad de México, Mexico, 1963; pp. 1–220. Available online: (accessed on 4 February 2021).
- Hoffmann, C.C.; Dampf, A.; Varela, G. Informe de la Comisión Científica Exploradora de la Plaga de la Langosta en el Estado de Veracruz, 1st ed.; Talleres Gráficos de México: Ciudad de México, Mexico, 1925; Volume 3, pp. 1–140.
- Ávila-Valdez, J.; Barrientos-Lozano, L.; García-Salazar, P. Aspectos de la Biología y hábitos de la langosta centroamericana, Schistocerca piceifrons piceifrons (Walker) (Orthoptera: Acrididae) bajo las condiciones del Sur de Tamaulipas. Entomol. Mex. 2007, 3, 177–181.
- Sword, G.A.; Simpson, S.J.; El Hadi, O.M.; Wilps, H. Density-dependent aposematism in the desert locust. Proc. Roy. Soc. B Biol. Sci. 2000, 267, 63–68.
- Pener, M.P.; Simpson, S.J. Locust phase polyphenism: An update. Adv. Insect Physiol. 2009, 36, 1–286.
- Song, H.; Foquet, B.; Mariño-Pérez, R.; Woller, D.A. Phylogeny of locusts and grasshoppers reveals complex evolution of density-dependent phenotypic plasticity. Sci. Rep. 2017, 7, 6606.
- Simpson, S.J.; Sword, G.A. Phase Polyphenism in Locusts: Mechanisms, Population Consequences, Adaptive Significance and Evolution. In Phenotypic Plasticity of Insects Mechanisms and Consequences; Whitman, D.W., Ananthakrishnan, T.N., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 147–189.
- Contreras-Servín, C. Conexión Climática del Fenómeno del Niño con la Plaga de la Langosta en Centroamérica (Schistocerca piceifrons piceifrons, Walker) Localizada en el Estado de Yucatán y la Huasteca Potosina. Entomol. Mex. 2009, 8, 34–351. Available online: (accessed on 5 February 2021).
- Senasica-DGSV. Central American Locust; Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Dirección General de Sanidad Vegetal: Ciudad de México, México, 2018; pp. 1–2. Available online: (accessed on 8 February 2021).
- Hernández-Velázquez, V.M.; Berlanga-Padilla, A.M.; Barrientos-Lozano, L. Vegetable and mineral oil formulations of Metarhizium anisopliae var. acridum to control the Central American Locust Schistocerca piceifrons piceifrons (Walker, 1870) (Orthoptera: Acrididae). J. Orthop. Res. 2000, 9, 223–227.
- Song, H.; Poot-Pech, M. Taller regional de manejo de la langosta centroamericana, Schistocerca Piceifrons. Metaleptea 2017, 37, 16–17.
- Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA). Situación Actual y Recomendaciones para el Control de la Langosta Centroamericana en los Países de la Región OIRSA, 1st ed.; Secretaría General del Sistema de la Integración Centroamericana: San Salvador, El Salvador, 2020; pp. 1–14. Available online: (accessed on 9 February 2021).
- Galván-Martínez, E.; Almaguer-Sierra, P.; Barrientos-Lozano, L. Dinámica poblacional de la langosta centroamericana, Schistocerca piceifrons piceifrons (Walker 1870), usando un modelo de simulación. Entomol. Mex. 2008, 7, 285–289. Available online: (accessed on 10 February 2021).
- Senasica-DGSV. Langosta Centroamericana. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Dirección General de Sanidad Vegetal. 2020. Available online: (accessed on 10 February 2021).
- Barrientos-Lozano, L.; Hernández-Velázquez, V.M.; Milner, R.J.; Hunter, D.M. Advances in biological control of locusts and grasshoppers in México. J. Orthop. Res. 2002, 11, 77–82.
- Hernández-Velázquez, V.M.; Berlanga-Padilla, A.M.; Garza-González, E. Detección de Metarhizium flavoviride sobre Schistocerca piceifrons piceifrons (Orthoptera: Acrididae) en la Isla Socorro, Archipiélago de Revillagigedo, México. Vedalia 1997, 4, 45–46.
- Barrientos-Lozano, L.; Milner, R.J. Comparison of the Pathogenicity of Three Isolates of Metarhizium anisopliae var. acridum, Effect of Dose and Temperature. In Proceedings of the XXI International Congress of Entomology, Foz de Iguaçu, Brazil, 20–26 August 2000; Sociedade Entomológica do Brasil: Londrina, PR, Brazil, 2000; pp. 513–514.
- Hernández-Velázquez, V.M.; Hunter, D.M.; Barrientos-Lozano, L.; Lezama-Gutiérrez, R.; Reyes-Villanueva, F. Susceptibility of Schistocerca piceifrons (Orthoptera: Acrididae) to Metarhizium anisopliae var. acridum (Deuteromycotina: Hyphomycetes): Laboratory and field trials. J. Orthop. Res. 2003, 12, 89–92.
- Milner, R.J.; Barrientos-Lozano, L.; Driver, F.; Hunter, D.M. A comparative study of two Mexican isolates with an Australian isolate of Metarhizium anisopliae var. acridum strain characterization, temperature profile and virulence for wingless grasshopper, Phaulacridium vittatum. BioControl 2003, 48, 335–348.
- Barrientos-Lozano, L.; Hunter, D.M.; Ávila-Valdéz, J.; García-Salazar, P.; Hernández-Velázquez, V.M. Control Biológico de la Langosta Voladora (Schistocerca piceifrons Walker) con Metarhizum anisopliae var. acridum en el Noreste de México. In Proceedings of the XXVII Congreso Nacional de Control Biológico, Los Mochis, Sinaloa, México, 8–13 November 2004; Sociedad Mexicana de Control Biológico: Sinaloa, Mexico, 2004; pp. 137–141. Available online: (accessed on 12 February 2021).
- Barrientos-Lozano, L.; Hunter, D.M.; Ávila-Valdéz, J.; García-Salazar, P.; Horta-Vega, J.V. Control Biológico de la Langosta centroamericana Schistocerca piceifrons piceifrons Walker (Orthoptera: Acrididae) en el Noreste de México. Vedalia 2005, 12, 119–128.
- Williams, T.; Arredondo-Bernal, H.C.; Rodríguez-del-Bosque, L.A. Biological Pest Control in Mexico. Annu. Rev. Entomol. 2013, 58, 119–140.
- Barrientos-Lozano, L. Manejo Integrado de la Langosta Centroamericana (Schistocerca piceifrons piceifrons, Walker) y Acridoideos Plaga en América Latina, 1st ed.; Tecnológico Nacional de México-Instituto Tecnológico de Cuidad Victoria: Cuidad Victoria, Tamaulipas, Mexico, 2005; pp. 1–303.
- Barrientos-Lozano, L. Memoria Simposio Control. In Biológico y Manejo de la Langosta Centroamericana Schistocerca piceifrons piceifrons (Walker); Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria-Instituto Tecnológico de Cuidad Victoria: Cuidad Victoria, Tamaulipas, Mexico, 2007; pp. 1–112.
