Chitin is mentioned as the second most abundant and important natural biopolymer in worldwide scale. The main sources for the extraction and exploitation of this natural polysaccharide polymer are crabs and shrimps. Chitosan (poly-β-(1 → 4)-2-amino-2-deoxy-d-glucose) is the most important derivative of chitin and can be used in a wide variety of applications including cosmetics, pharmaceutical and biomedical applications, food, etc., giving this substance high value-added applications. Moreover, chitosan has applications in adsorption because it contains amino and hydroxyl groups in its molecules, and can thus contribute to many possible adsorption interactions between chitosan and pollutants (pharmaceuticals/drugs, metals, phenols, pesticides, etc.). However, it must be noted that one of the most important techniques of decontamination is considered to be adsorption because it is simple, low-cost, and fast.
- chitosan
- synthesis
- characterization
- adsorption capacity
1. Chitosan
Emerging contaminants (ECs) may be defined as compounds that are not currently covered by existing water regulations but are thought to be a threat to environmental ecosystems and human beings. The presence of pharmaceuticals, even in low concentrations, constitute a danger to human and animal and to aquatic species. The widespread incidence of pharmaceuticals in water brings into light the inadequacy of conventional methods of water treatment and the necessity of developing alternative technologies for the optimization of the removal process. Particularly, the pharmaceutical compounds have been found in all aquatic environments (river, lakes, etc.), groundwater, and wastewater plant effluents in several countries, making it a significant environmental issue [1].
Chitosan (poly-β-(1→4)-2-amino-2-deoxy-d-glucose), the second most abundant biopolymer in nature after cellulose, is a polysaccharide produced by N-deacetylation of chitin [2]. Chitin exists in marine media and especially in the exoskeleton of crustaceans, or cartilages of mollusks, cuticles of insects and cell walls of microorganisms. Chitosan is a promising material not only for the remarkable properties such as biodegradability, biocompatibility, and antimicrobial activity but also for its adsorption capacity, as its cationic character enable it to interact with other molecules. The presence of amino and hydroxyl groups on the structure makes possible the chemical modification of chitosan with the purpose of improving its solubility and electric change [3]. Furthermore, the modification of chitosan aims to ameliorate the surface area, hydrophilicity, and hydrolysability in acidic conditions, which remain some of the main disadvantages of this biopolymer. In the case of adsorption, which is considered the most promising separation technique [4][5][6][7][8][9][10], the modification of chitosan/or its composites is expected to fabricate materials, mainly with high surface area, mechanical strength, and functional groups for binding with the contaminants [11].
2. Synthetic Routes and Characterizations of Chitosan
2.1. Chitosan/Modified Chitosan Beads
One acceptable form of chitosan (CS) used for the removal of pharmaceutical compounds is CS beads using the method of insoluble gelation. Studies showed that CS gelation beads (via adding chitosan solution to an alkaline non-solvent) is an efficient way to separate adsorbents with high affinity to contaminants. However, in order to enhance the acid resistance and mechanical properties, cross-linkers such as glutaraldehyde (GA), epichlorohydrin (EP), and glycol diglycidyl ether were used, which also influences the adsorption capacity. Lu et al. propose the utilization of chitosan beads grafted by polyethylenimine (PEI) and further cross-linked with glutaraldehyde and epichlorohydrin for the removal of diclofenac sodium (DS) from water. Sharper peaks of modified beads on spectrum of Fourier-transform infrared spectroscopy (FT-IR) revealed the successful synthesis with the introduction of more functional groups. It is remarkable that CS beads after grafting and crosslinking became more impenetrable to light and more stable. Regarding the surface of epichlorohydrin–polyethylenimine (EPCS@PEI), beads were rougher and smaller compared to unmodified CS beads, attributed to the presence of PEI. By comparison, CS beads grafted with glutaraldehyde and PEI grafted (GACS@PEI) were much smoother, which is connected to the lower adsorption capacity (Figure 1) [12].
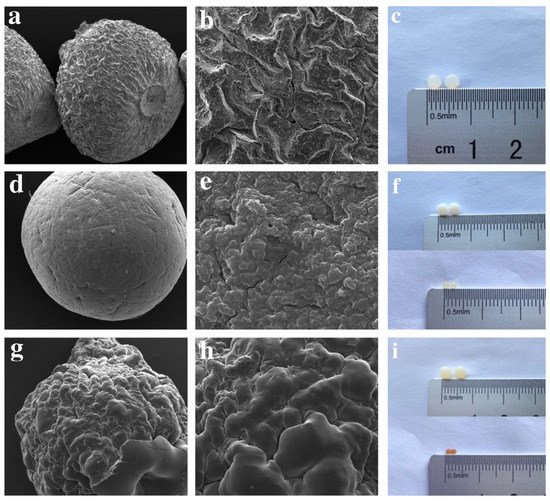
Figure 1. SEM images of (a,b) CS, (d,e) EPCS@PEI, (g,h) GACS@PEI beads. Photographs of wet and dried beads of (c) CS, (f) EPCS@PEI, and (i) GACS@PEI [12].
Another work focused on tricaprylmethylammonium chloride CS hydrogel beads (CS-TCMA) for the fast adsorption of tetracycline (TC). TCMA has been demonstrated to enhance the adsorption capacity of TC, which is known as an ion-pairing reagent. The whole procedure, according to the research, lasts less than 45 min and has a 90% yield. This is very promising, considering the fact that tetracycline (TC) is the second-most produced and used antibiotic (cheap and high antimicrobial activity). Moreover, FTIR spectroscopy confirmed the incorporation of the TCMA on CS as well as the interaction between the hydrogel beads and the drugs. Scanning electron microscope (SEM) images reveal a dispersion of the TCMA on CS, concerning the homogenous porous structure of the composite [13].
2.2. Chitosan Nanoparticles/Chitosan Film
Many researchers have worked on chitosan nanoparticles, especially for drug delivery applications. However, another potential application is their use in water purification. In general terms, the techniques of preparation and the kind of cross-linking plays an important role to the properties of the final product. The research group of Riegger suggested emulsion cross-linking and described the impact of cross-linker concentration and the molecular weight of six commercial chitosans on chitosan nanoparticle formation. The best ratio found for glutaraldehyde: primary amino groups, in order to be narrowly distributed, covalent cross-linked, and form nanoscale chitosan particles, was found to be 1:1. Generally, covalent crosslinking is desired in the case of regeneration processes with strong pH changes, as the resulting products show a high chemical and thermal stability. Prepared emulsions had an aqueous phase, chitosan solutions and an oil phase Span 80, and then NaCl was used to further stabilize the system (Figure 2). The adsorption behavior of the products was tested for two drugs: diclofenac (DCF) and carbamazepine (CBZ). Results from CBZ showed that untreated chitosans have lower adsorption than nanoparticles, while the lack of charge in CBZ is also associated with level of adsorption. In the case of DCF, there is the same tendency between the untreated chitosans and the nanoparticles. The study shows that smaller particles and better surface to volume ratio enhance the adsorption. For that reason, chitosan nanoparticles tend to have better results compared to untreated material. Broadly speaking, this system seems to be an interesting option for the treatment of drug-contaminated drinking water [14].
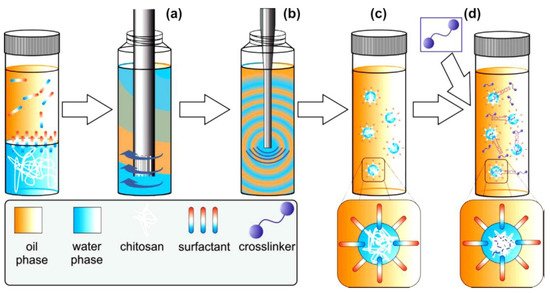
Figure 2. Schematical representation of emulsion crosslinking technique [14]. In a first step, the continuous phase was premixed with the disperse phase by rotor-stator treatment (a) followed by ultrasonication (b). To the resulting white and optically opaque emulsion (c) the crosslinker glut was added (d). To complete the crosslinking reaction, the emulsion was stirred for 18 h.
Rizzi et al. examined the removal of furosemide, one of the most dangerous pharmaceuticals, which causes hepatotoxicity and ototoxicity to aquatic species, from chitosan film. In addition, furosemide is associated with the development of toxic metabolites, even forced by its fractions. As a result, it is a challenge to find an adsorbent that is suitable for furosemide removal from waters and economically beneficial. Particularly, the adsorption was achieved due to interactions between the protonated amino groups of chitosan and the carboxyl groups of the drug molecule. A quite low adsorption capacity is presented (3.5 mg/g), with the aim of inorganic salt of sodium chloride (NaCl 1M) desorption of 90% of adsorbed furosemide performed, suggesting both the reuse of the pollutant and recycling of the adsorbent for more repetitions [15].
2.3. Grafted Chitosan
According to the fact that the adsorption of pharmaceuticals is depending also on the chemical structure of the biopolymer, it is of great importance to have enough sites available for chemical binding with the pollutants. This can happen with the grafting of chitosan and thus the introduction of extra functional groups. Kyzas et al. synthesized modified chitosan with sulfonic acid and cross-linked it with glutaraldehyde to examine the impact of humic acid in the whole process. Humic acid (HA) corresponds to the mixture of different acids and is a major compound of natural organic matter (NOM) and product of biodegradation of dead organic matter. As a model pharmaceutical pollutant, pramipexole dihydrochloride (PRM) was used. Drying method caused changes to the structure of the modified material, perhaps because of water molecules preexisting there. Broadly, the innovation of this study is supported by the coexistence of humic acid on the adsorbate pramipexole (PRM) in various concentrations. Results showed that the increase in the concentration of HA in water is associated with the decrease in the maximum adsorption capacity of pharmaceuticals, as there is a crucial concentration of HA at 5 mg/L. The same group also examined graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite (GO/CSA) for its adsorption strength. FT-IR spectra showed that the existence of amino groups, because of basic conditions of preparation of the composite, can cause amine nucleophilic substitution on the epoxy groups of GO. The introduction of chitosan on GO cn be indicated by the absence of C=C absorbance due to the carbon structure network. The composite material appears more adsorption efficiency than neat graphite oxide or poly(acrylic acid) grafted chitosan. Precisely, cationic groups of dorzolamide interacted with anionic groups of the derivatives while crosslinking with glutaraldehyde to increase the resistance in a wide range of pH. As a target drug, they used dorzolamide, a substance used for ocular release. It must be noted that the adsorption capacity was increased in a temperature range of 25–65 °C. It is remarkable that on FTIR spectroscopy of the adsorbent with dorzolamide, we can see the reduced peak of carbonyl groups of CSA and a new peak at 1622 cm−1, which may be attributed to the amide I formation due to interactions between charged carboxyl groups of CSA and charged amino groups of the drug. There is a considerable literature from Kyzas’ group on the subject, as they also studied modified chitosan with sulfonate (CsSLF) or N-(2-carboxybenzyl) groups (CsNCB) and crosslinked with glutaraldehyde in an effort to remove pramipexole dihydrochloride (PRM). It was found that alkaline conditions contribute to maximum adsorption while acidic conditions to maximum desorption. Results showed that modification enhanced the adsorption capacity while CsSLF present a better adsorption effect than CsNCB. The morphology of modified materials is not as smooth as the neat chitosan while CsSLF is rougher than CsNCB. The damage of the porosity of the material after modification showed affect the particle size of the materials/surface area. FTIR confirmed the modification although new peaks appeared due to the existence of homopolymers on the material. As a result, the two grafted materials are proposed as adsorbents with low cost and ecological perspective [16].
Another study from Tzereme et al. examined the adsorption capacity of four different grafted chitosans with succinic anhydride (CsSUC), maleic anhydride (CsMAL), itaconic acid (CsITA), and trans-aconitic acid (CsTACON) towards pharmaceutical compound diclofenac (DCF) and mixture of salicylic acid, ibuprofen, and ketoprofen. All materials were cross-linked with glutaraldehyde. The acidic condition of the process also facilitates the electrostatic attraction of cation amino groups of chitosan and negative carboxyl moieties of chitosan derivatives. In FTIR spectroscopy, new peaks appear at 1700–1740 cm−1 and also a lower intensity of –NH2 peaks, facts that confirm the successful modification. Adsorption was carried out with several solvents [17].
Another research group used as an adsorbent modified chitosan with acrylic monomer, 2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide in order to remove a mixture of anti-inflammatory drugs (diclofenac, ibuprofen, ketoprofen, paracetamol, and salicylic acid). The modification was prepared via free radical polymerization and the final material was further cross-linked with glutaraldehyde. Due to the coexistence of sulfonate anion groups and quaternary ammonium cations, the system interacts primarily with ionic forces and then with hydrogen bonds with the drug molecules. These interactions were also confirmed with FTIR spectroscopy. In conclusion, SEM images showed even smaller external pores than before adsorption.
2.4. Chitosan Composites with Magnetic Properties
Concerning the magnetic separation, this kind of separation is fast, expandable, easily automated, and can achieve complete separation compared to other techniques [18].
Ahamad and his group synthesized a new magnetic polymeric nanocomposite containing magnetic nanoparticles (MnFe3O4) (CDF@MF) and studied its application as adsorbent for tetracycline (TC). FT-IR study exhibited the successful synthesis of the composite material, concerning the characteristic absorption peaks. High surface area and favorable porous volume offers a promising substructure to the composite for more sites for adsorption. It seemed that CDF@MF had promising magnetic behavior that encourages the recyclability of the material after adsorption. Finally, SEM images showed the dispersion of the magnetic nanoparticles in the polymer matrix while the small size helps the adsorption of TC [19].
Zhang et al. referred to grafting co-polymerization on the surface of chitosan/Fe3O4 particles (CS-MCP). The aforementioned composite forms a core-brush structure, where the neat MCP constitute the core and the modified polymeric branches make up the brush layer. FT-IR measurements of the material demonstrated both –NH2 and Fe-O absorptions. The modification was carried out to add functional groups to neat MCP and thus help the adsorption process of organic pollutants. Diclofenac sodium (DCM) tetracycline hydrochloride was selected as an emerging organic contaminant. Enhanced removal capacity is due to the surface area available and core-brush topology and the interactions among positively charged polymeric branches and negatively charged groups of the pharmaceutical compounds (Figure 3) [20].
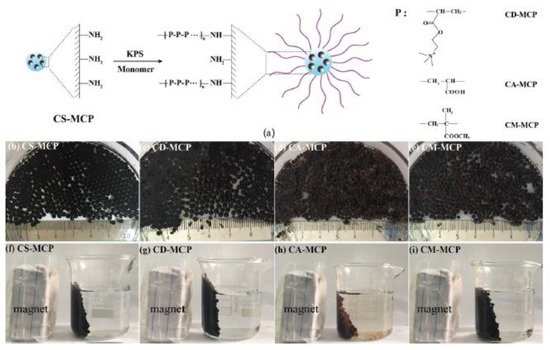
Figure 3. (a) Synthetic route, (b–e) photos, and (f–i) magnetic separation property of different adsorbents in water at room temperature [20].
Zhou et al. suggested core-brush structure containing chitosan and Fe3O4 composite particles (CS-MCPs) for the adsorption of commonly used norfloxacin (NOR), tylosin (TYL), and diclofenac sodium (DCF). They state that the most preferable process for the modification of MCP, in order to introduce extra functional groups, is a core of MCP and post-modification on the branches, which can easier contact the contaminant. FTIR spectra showed peaks at 1637 cm−1 and 609 cm−1, referring to –NH2 and Fe–O, respectively. In total, in this research, they tried to study the effect of brush modification of CS-MCP with polystyrene derivatives for the removal of pharmaceuticals. Concretely, modification with poly (sodium p-styrenesulfonate) enhanced the adsorption efficiency of NOR and TYL while modification with (poly-p-vinylbenzyl trimethylammonium chloride) successfully adsorbed DCF. In all cases, modification helped the pH resistance, in a narrow range of values, and also resulted in reusable systems (six cycles of processes). It is remarkable that after modification, the surface of CS-MCP becomes rough from smooth, and has better BET surface area. (Figure 4) [21].
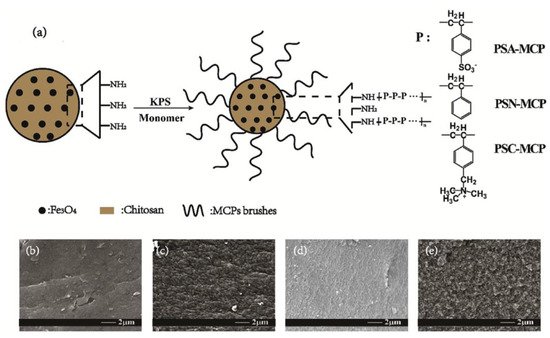
Figure 4. (a) Synthetic route; SEM images of (b) CS-MCP, (c) PSA-MCP, (d) PSN-MCP, and (e) PSC-MCP [21].
Liu used chitosan/graphene oxide-SO3H composite (GC/MGO-SO3H) with super paramagnetic behavior for the removal of ibuprofen and tetracycline. In the case of MGO-SO3H, FTIR spectroscopy showed the characteristic peak of MGO-SO3H at 560 cm−1, while in the case of GC/MGO-SO3H, hydrogen bonding between chitosan and GO is confirmed. The composite cross-linked with genipin for further stability. Chitosan magnetic composite has high congestion magnetization and magnetic permeability and graphene-based materials large surface area. In general, salts of chitosan magnetic composite could be merged with graphene oxide (GO) by electrostatic interaction. Precisely, the sulfa group is known to form stable complexes with various pharmaceuticals. Furthermore, the microporous structure with an ultra-large surface area enhanced the adsorption of ibuprofen and tetracycline drugs. The benefit of the hybrid is the ability to reuse it as it maintains the adsorption capacity at 85% after 5 cycles [22].
2.5. Chitosan Combined with MOFs
Another view of the subject gave Zhuo and his group using MOFs. In order to improve the ability of separation of neat metal organic frameworks (MOFs), they prepared MIL-101(Cr)/chitosan (MIL-101 (Cr)/CS) composite beads for the removal of ibuprofen (IBU) and ketoprofen (KET). A peak at 589 cm−1 on FTIR spectra prove the formation of the MOF, while the characteristic peaks of chitosan are shown, too. Compared to neat CS beads, the composite beads appear to have better adsorption capacity with a larger amount that can be withheld in the case of ketoprofen. Both element chromium (Cr) and protonated amino groups of chitosan help the conjunction with the pollutant, which was evaluated with X-ray photoelectron spectroscopy (XPS). Finally, the great regenerability composite beads appear, making them possible candidates for large-scale water treatment [23]. In another study, Jia et al. prepared a starch-chitosan-UiO-66-COOH composite as an adsorbent for pharmaceutical sulfonamide. Results showed hierarchical porosity of the composite and also efficient removal of the drug task. Chitosan seemed to facilitate the binding between starch and the MOF, proven also from FTIR spectra, and as well to decrease the aggregations of MOF nanoparticles during formulation. Zr-O bond of Zr-MOF with sulfonic groups of sulfonamide is the main reason of the efficient removal. The easy synthesis and the low cost are some advantages of the material [24].
2.6. Other Chitosan Composites
In the field of GO/CS composite, the research group of Delhiraja studied the composite material of activated carbon (AC), graphene oxide (GO), and chitosan (GO-AC-CS), combining the hydrophilicity, hydrophobicity, and binding properties of each component, respectively. A decrease in the intensity of transmittance in the carboxyl group region, in the case of GO-based composites, is connected to the new bond between the hydroxyl group of GO and the ring of chitosan. As pharmaceutical pollutants, they used acetaminophen (ACP) and carbamazepine (CBZ). The group took advantage of modified Hummer’s method in order to obtain GO with many oxygen-containing functional groups. According to the results, the system shows a behavior sensitive to pH and organic matter. Concerning the morphology, SEM images showed clusters, flakes, and small ball-like formations for neat GO, AC, and CS, respectively. On the contrary, GO-CS appears as smooth clusters, confirming the bonding between the two. Figure 5 shows the insertion of chitosan between the crystal network of graphene oxide. Regeneration experiments showed that with appropriate organic solvent, the removal of the 80% of adsorbed matter can be achieved [25].
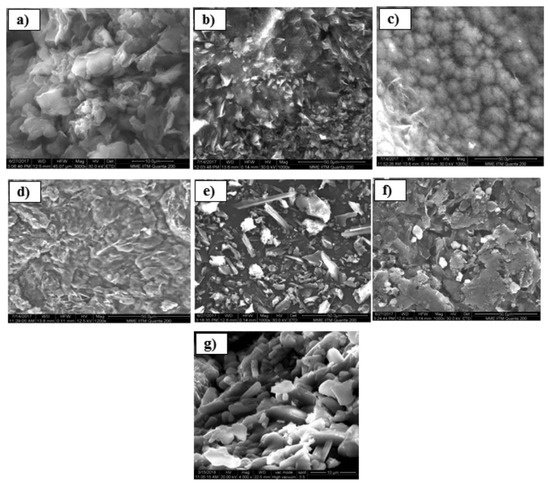
Figure 5. SEM images of the synthesized pristine and composite adsorbents (a) GO, (b) AC, (c) CS, (d) GO-CS, (e) AC-CS, (f) GO-AC-CS, and (g) cross-sectional SEM image of GO-AC-CS [25].
Other researchers examined the multifunctional combination of chitosan-EDTA-β-cyclodextrin (CS-ED-CD) for the deportation of ciprofloxacin, procaine, and imipramine from effluents. FTIR spectra of the composite showed new peaks compared to raw materials, due to carbonyl groups of amides formed and carboxylic groups inserted. The selection of EDTA as a cross-linker attracts interest because of its low cost and low toxicity compared to common cross-linkers. Likewise, chitosan contributes to higher loading of CD SEM images, indicating a thin, porous layer on the surface and a continuous porous internal morphology with pore sizes from 20 to 200 μm. Pore size measurements from SEM and BET (Figure 6) are incompatible, but this a consequence of the different magnitude of dimensions in each method (pores sized less than 250 nm on BET and micrometers on SEM). As is expected on most biopolymers, the surface area and the porosity of the prepared complex were not notably higher, but that does not really influence the adsorption process, which mainly depends on the functional groups incorporated on the biopolymers [26].
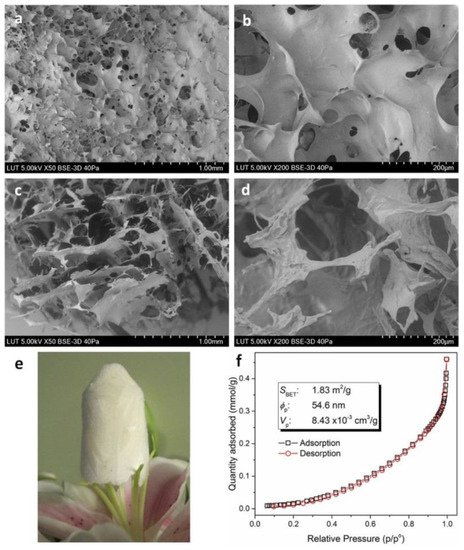
Figure 6. SEM images of freeze-dried CS-ED-CS polymer. Surface morphologies (a,b) and cross-sectional morphologies (c,d) of CS-ED-CD hydrogel with varying magnifications; a block of freeze-dried CS-ED-CD hydrogel standing on the stamens of a lilium flower (e); and BET isotherm linear plot and BET surface area (f), average pore diameter and cumulative pore volume data for CS-ED-CD [26].
Lessa et al., in order to adsorb from wastewaters metamizol (MET), acetylsalicylic acid (ASA), and acetaminophen (ACE) used a waste coffee grounds (WCG)-chitosan-poly (vinyl alcohol) (WCG-CA-PVA) composite. The concentration of 10% WCG in the system seems to be effective for the formulation, adding enough adsorption sites. SEM images indicate that increase in the WCG percentage provoke rougher surfaces and some aggregates, perhaps because there is a destruction of the compatibility of the polymer matrix and the filler. The main advantage of this research is the cost-beneficial process using the aforementioned waste and also the reusability in at least five cycles [27].
Regarding the important role of the size of the particles on adsorbent materials, Feng took advantage of the spray drying process in order to prepare, with a low cost, chitosan microparticles to acquire larger surface area. Particularly, he indicates the use of chitosan/nanographene oxide (CS/nGO) shows high adsorption efficiency of the anti-inflammatory drug diclofenac sodium (DCF). The 100% adsorption seems to be connected to coexisting hydrogen bonding and the electrostatic interactions among the positive charged amino groups and the anions of the compounds. They presented that the addition of nGO promote the reusability of the microspheres with 80% capacity of adsorption of DCF after six cycles of action [28]. With the same concept as Feng, Yanyan et al. investigated, among others, the use of modified multi-walled carbon nanotubes (MWCNTs) with chitosan coating for the adsorption of acetaminophen (Ace) from wastewater, in order to ameliorate the attachment of the model drug. It seems that chitosan coating in MWCNT also filled the interval between nanotubes. Specifically, in the case of chitosan coated MWCNTs, a new peak at 1630 cm−1 seems to reveal the presence of chitosan. Nevertheless, the treatment of MWCNT with ozone seems to be more efficient for the adsorption of acetaminophen, perhaps due to the presence of extra hydroxyl and carboxyl groups. At the same time, even more bioprocesses are required to achieve the limit of less than 0.2 mg/L, as reported by Chinese regulations [29].
This entry is adapted from the peer-reviewed paper 10.3390/macromol1020011
References
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127.
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpää, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631.
- Samadi, F.Y.; Mohammadi, Z.; Yousefi, M.; Majdejabbari, S. Synthesis of raloxifene-chitosan conjugate: A novel chitosan derivative as a potential targeting vehicle. Int. J. Biol. Macromol. 2016, 82, 599–606.
- Fu, J.; Kyzas, G.Z.; Cai, Z.; Deliyanni, E.A.; Liu, W.; Zhao, D. Photocatalytic degradation of phenanthrene by graphite oxide-TiO2-Sr(OH)2/SrCO3 nanocomposite under solar irradiation: Effects of water quality parameters and predictive modeling. Chem. Eng. J. 2018, 335, 290–300.
- Kyzas, G.Z.; Nanaki, S.G.; Koltsakidou, A.; Papageorgiou, M.; Kechagia, M.; Bikiaris, D.N.; Lambropoulou, D.A. Effectively designed molecularly imprinted polymers for selective isolation of the antidiabetic drug metformin and its transformation product guanylurea from aqueous media. Anal. Chim. Acta 2015, 866, 27–40.
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811.
- Anastopoulos, I.; Hosseini-Bandegharaei, A.; Fu, J.; Mitropoulos, A.C.; Kyzas, G.Z. Use of nanoparticles for dye adsorption: Review. J. Dispers. Sci. Technol. 2018, 39, 836–847.
- Kyzas, G.Z.; Lazaridis, N.K.; Kostoglou, M. On the simultaneous adsorption of a reactive dye and hexavalent chromium from aqueous solutions onto grafted chitosan. J. Colloid Interface Sci. 2013, 407, 432–441.
- Anastopoulos, I.; Kyzas, G.Z. Composts as biosorbents for decontamination of various pollutants: A review. Waterairand Soil Pollut. 2015, 226.
- Terzopoulou, Z.; Papageorgiou, M.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Preparation of molecularly imprinted solid-phase microextraction fiber for the selective removal and extraction of the antiviral drug abacavir in environmental and biological matrices. Anal. Chim. Acta 2016, 913, 63–75.
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 291.
- Lu, Y.; Wang, Z.; Ouyang, X.-k.; Ji, C.; Liu, Y.; Huang, F.; Yang, L.-Y. Fabrication of cross-linked chitosan beads grafted by polyethylenimine for efficient adsorption of diclofenac sodium from water. Int. J. Biol. Macromol. 2020, 145, 1180–1188.
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Khadempir, S.; Sillanpää, M. Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol. 2020, 155, 421–429.
- Riegger, B.R.; Bäurer, B.; Mirzayeva, A.; Tovar, G.E.M.; Bach, M. A systematic approach of chitosan nanoparticle preparation via emulsion crosslinking as potential adsorbent in wastewater treatment. Carbohydr. Polym. 2018, 180, 46–54.
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Nuzzo, S.; Gabaldón, J.A.; Gorbe, M.I.F.; Gómez-Morte, T.; Cosma, P. Chitosan film as recyclable adsorbent membrane to remove/recover hazardous pharmaceutical pollutants from water: The case of the emerging pollutant Furosemide. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 56, 145–156.
- Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Effect of humic acid on pharmaceuticals adsorption using sulfonic acid grafted chitosan. J. Mol. Liq. 2017, 230, 1–5.
- Tzereme, A.; Christodoulou, E.; Kyzas, G.Z.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures. Polymers 2019, 11, 497.
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Lazaridou, M.; Bikiaris, D.N.; Yang, X.; Kyzas, G.Z.; Lambropoulou, D.A. Simultaneous removal of anti-inflammatory pharmaceutical compounds from an aqueous mixture with adsorption onto chitosan zwitterionic derivative. Colloids Surf. A Physicochem. Eng. Asp. 2021, 126498.
- Ahamad, T.; Ruksana; Chaudhary, A.A.; Naushad, M.; Alshehri, S.M. Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol. 2019, 134, 180–188.
- Zhang, S.; Dong, Y.; Yang, Z.; Yang, W.; Wu, J.; Dong, C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 2016, 304, 325–334.
- Zhou, X.; Dong, C.; Yang, Z.; Tian, Z.; Lu, L.; Yang, W.; Wang, Y.; Zhang, L.; Li, A.; Chen, J. Enhanced adsorption of pharmaceuticals onto core-brush shaped aromatic rings-functionalized chitosan magnetic composite particles: Effects of structural characteristics of both pharmaceuticals and brushes. J. Clean. Prod. 2018, 172, 1025–1034.
- Liu, Y.; Liu, R.; Li, M.; Yu, F.; He, C. Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohydr. Polym. 2019, 220, 141–148.
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280.
- Jia, X.; Zhang, B.; Chen, C.; Fu, X.; Huang, Q. Immobilization of chitosan grafted carboxylic Zr-MOF to porous starch for sulfanilamide adsorption. Carbohydr. Polym. 2021, 253, 117305.
- Delhiraja, K.; Vellingiri, K.; Boukhvalov, D.W.; Philip, L. Development of Highly Water Stable Graphene Oxide-Based Composites for the Removal of Pharmaceuticals and Personal Care Products. Ind. Eng. Chem. Res. 2019, 58, 2899–2913.
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7.
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266.
- Feng, Z.; Danjo, T.; Odelius, K.; Hakkarainen, M.; Iwata, T.; Albertsson, A.C. Recyclable Fully Biobased Chitosan Adsorbents Spray-Dried in One Pot to Microscopic Size and Enhanced Adsorption Capacity. Biomacromolecules 2019, 20, 1956–1964.
- Yanyan, L.; Kurniawan, T.A.; Albadarin, A.B.; Walker, G. Enhanced removal of acetaminophen from synthetic wastewater using multi-walled carbon nanotubes (MWCNTs) chemically modified with NaOH, HNO3/H2SO4, ozone, and/or chitosan. J. Mol. Liq. 2018, 251, 369–377.
