Traditional influenza vaccines generate strain-specific antibodies which cannot provide protection against divergent influenza virus strains. Further, due to frequent antigenic shifts and drift of influenza viruses, annual reformulation and revaccination are required in order to match circulating strains. Thus, the development of a universal influenza vaccine (UIV) is critical for long-term protection against all seasonal influenza virus strains, as well as to provide protection against a potential pandemic virus. One of the most important strategies in the development of UIVs is the selection of optimal targeting antigens to generate broadly cross-reactive neutralizing antibodies or cross-reactive T cell responses against divergent influenza virus strains. However, each type of target antigen for UIVs has advantages and limitations for the generation of sufficient immune responses against divergent influenza viruses.
- influenza
- universal vaccine
- antigen
- immune response
Note: The following contents are extracted from your paper. The entry will be online only after author check and submit it.
1. Introduction
2. HA-Based UIVs
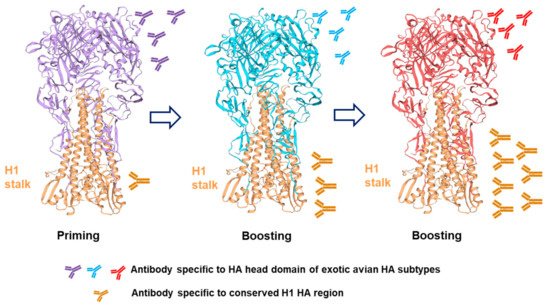
2.1. Headless HA
2.2. Chimeric HA
| Immunogen | Vaccine Name /Platform |
Identifier | Manufacturer | Phase | Status | Effect | Adjuvant |
|---|---|---|---|---|---|---|---|
| HA | Chimeric HA-based LAIV combinations | NCT03300050 | Icahn School of Medicine at Mount Sinai (US) | 1 | Completed | Induces high anti-stalk Ab titers and long-lasting immunity | AS03A |
| M2e | M2e-based VLPs | NCT00819013 | Ghent Univ (Belgium) Sanofi Pasteur (US) |
1 | Completed | Induces anti-M2e Ab | Alum and QS-21 |
| RedeeFlu M2SR/M2e-deficient | NCT03999554 NCT02822105 NCT03553940 NCT04785794 |
Flugen, Inc. (US) | 1 1 1 1 |
Completed Completed Completed Ongoing |
Reduces symptom scores and virus replication | None | |
| NP and M1 | MVA−NP+M1/Viral vector | NCT00942071 NCT00993083 NCT01465035 NCT01818362 NCT02014168 NCT03277456 NCT03300362 NCT03883113 NCT03880474 |
Vaccitech Ltd. | 1 2 1 1 1 1 2 2 2 |
Completed Completed Completed Completed Terminated Completed Completed Completed Terminated |
Reduces influenza symptoms and length of virus shedding, induces T cell responses |
None |
| ChAdOx1 NP+M1/Adenoviral vector | NCT01623518 | Jenner Institute, University of Oxford | 1 | Completed | Increases T cell response | None | |
| NP | OVX836/Reco-mbinant NP | NCT04192500 NCT03594890 | Osivax SAS (France) | 2 1 |
Completed Completed |
None yet reported | None |
| HA, NP, and M1 | M-001/ Recombinant protein | NCT01010737 NCT01146119 NCT01419925 NCT02293317 NCT02691130 NCT03058692 NCT03450915 |
BiondVax Pharmaceuticals Ltd. (Israel) | 1/2 2 2 2 2 2 3 |
Completed Completed Completed Completed Completed Completed Completed |
Induces significant cellular-mediated immunity and HI titers | Montanide ISA-51/ Oil –in-water |
| NP, M1, P1, and P2 | FP-01.1/ Peptide-based |
NCT01265914 NCT01677676 NCT01701752 NCT02071329 |
Immune Targeting Systems Ltd. (United Kingdom) | 1 1 1 1/2 |
Completed Completed Completed Completed |
Good safety and tolerability profiles | None |
| NP, M1, and M2 | FLU-v/ Peptide-based | NCT01181336 NCT01226758 NCT03180801 NCT02962908 |
PepTcell (SEEK, United Kingdom) | 1 1 2 2 |
Completed Completed Completed Completed |
Stimulates cell-mediated immunity, reduces symptomatology and virus shedding | Montanide ISA-51/Oil –in-water |
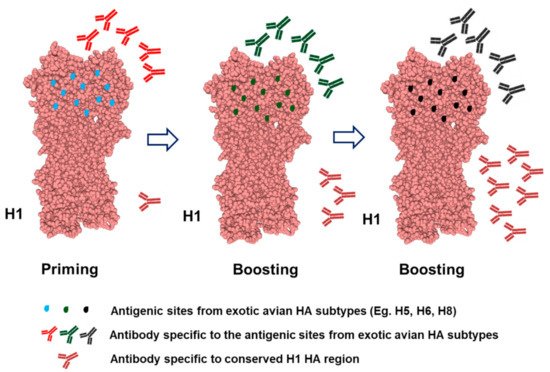
2.3. Mosaic HA
2.4. COBRA HA
2.5. “Breathing” HA
3. NA-Based UIVs
4. M2e-Based UIVs
5. Internal Proteins-Based UIVs
6. Multiple Proteins/Peptides-Based UIVs
7. Influenza Vaccine Adjuvants
8. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/v13060973
