Laboratory diagnostics which compatible with critical laboratory equipment are conceptually easily applied to patients, given the features of the following multiple operations of virus testing [
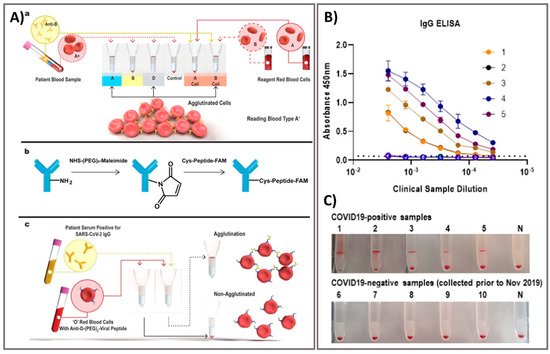
71]. Exposure tracking can limit the viral spread, however, population screening to determine virus infection levels in the community is a longer-term need. For lab-in-a-tube, Alves et al. 2020 built a sensing platform using gel tag agglutination tests to target SARS-CoV-2 with rapid case identification (). Ten serological samples in both gel cards and indirect IgG ELISA were tested and showed that similar performance between them, suggesting this assay as one of suitable approach for clinical diagnosis compared to conventional ELISA, owing to its advantages in excellent resolution and benefits of high throughput, high speed (10–30 min), automatic in most cases, and possibility for POC diagnostics. From gel card agglutination assays to lab-in-a-tube systems, this simple, rapid, and scalable approach could identify disease-specific activity to apply in SARS-CoV-2 testing, suggesting the ability to move diagnostics to the POC test for COVID-19 confirmation [
62]. In addition, Lin et al. 2020 recently demonstrated real-time and continuous monitoring platform using integrated diagnostic microchips, homemade mobile fluorescence detectors, and microfluidic immunoassay systems for the simultaneous detection of IgG/IgM/antigen in SARS-CoV-2. This system was utilized for SARS-CoV-2 serological testing, displaying high accuracy not only in distinguishing between infected and uninfected cases but also in determining the severity the disease, allowing disease staging as follows: stage 1 (infected 1–7 days), stage 2 (infected 8–14 days), and stage 3 (infected over 14 days). Furthermore, this system showed excellent sensor characteristics with a rapid response time of 15 min [
63]. For case of electrochemical sensor, Vadlamani et al. 2020 designed an electrode composed of cobalt-functional TiO
2 nanotubes (Co-TNT) as electrochemical sensor for the rapid detection of SARS-CoV-2 at low concentration range from 14–1400 nM with LOD of ~0.7 nM. The authors then used this system for real-time concentration measurements, showing a linear response in detecting viral proteins within concentration range for approximately 30 s in saliva and nasal secretions. The sensitivity of this approach can also be improved by using longer Co-TNTs due to higher surface area results in higher response rates, thus higher electric current can be obtained even at lower protein concentrations [
64]. Furthermore, an advanced nanomaterial-based electrochemical biosensor to detect SARS-CoV-2 antibodies within seconds was successfully developed to enhance the rapid diagnosis of COVID-19 for the better treatment and prevention of diseases [
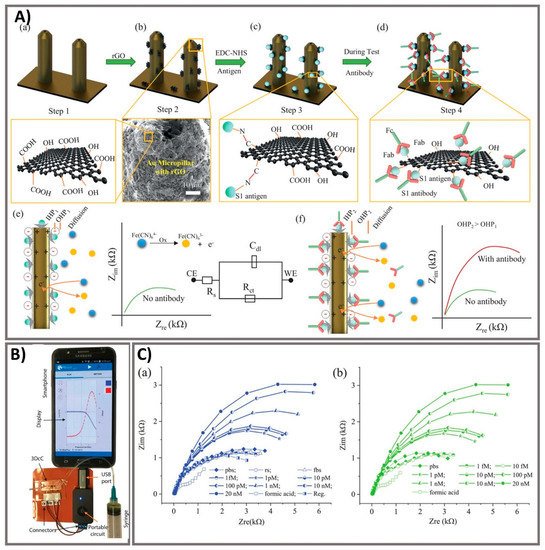
65]. The three-dimensional (3D) electrodes were printed using 3D nanoprinting and were coated with nanoflakes of reduced-graphene-oxide (rGO); specific viral antigens were then immobilized on the rGO nanoflakes. The electrode was then integrated with a microfluidic device and applied as an electrical immunosensor. In the presence of antibodies against the SARS-CoV-2 S1 protein, the antibodies selectively bound to the antigens due to their strong immunoaffinity, leading to a change in impedance of the electrical circuit, which is detected via impedance spectroscopy. Antibodies to SARS-CoV-2 S1 protein and its receptor-binding domain were detected by a smartphone-based user interface within 10 s with a wide concentration range from 1 fM to 20 nM at LOD of 2.8 × 10
−15 and 16.9 × 10
−15 M, respectively (). Seo et al. 2020 developed the field-effect transistor based biosensing device with the support of antibody functionalized graphene sheets to detect SARS-CoV-2 protein in human nasopharyngeal swab specimens. This platform helped improve nano-sensor performance in clinical samples for over 1 min without significantly altering the sensing capacity to detect SARS-CoV-2 without requiring sample pretreatment or labeling. This device showed good sensitivity for the detection of SARS-CoV-2 spike protein at the concentration of 1 fg/mL in phosphate-buffered saline and 100 fg/mL in clinical transport medium. Moreover, the device could detect SARS-CoV-2 in the culture medium with LOD of 1.6 × 10 pfu/mL and clinical samples with LOD of 2.42 × 10
2 copies/mL, respectively. This fully reversible modular sensing platform is a viable candidate for continuous clinical monitoring [
61]. Overall, these biosensing platforms mentioned above exhibits high sensitivity, selectivity, and the rapid ability of monitoring SARS-CoV-2 for application in diagnosis of COVID-19. However, it highly requires the technical professions and instruments to conduct the sensing process that could ensure the accurate diagnosis. The important mission for researchers is to achieve proper stability, remove unwanted noise, and make the products commercially applicable after conducting a successful clinical trial.
Figure 4. Rapid agglutination assays as serological testing for the detection of antibodies against SARS-CoV-2. (
A) Schematic illustration of blood typing column agglutination test (CAT) with the brief antibody-peptide bioconjugates to produce the SARS-CoV-2 serological assay. (
a) Pipette a mixture of reagent red blood cells (RRBCs) with patient samples onto a gel card containing separation media, followed by incubation of the card for 5–15 min. (
b) The bioconjugation procedure to produce the antibody-peptide in two steps. (
c) Antibody-peptide-coated RRBCs were incubated with a patient sample on a neutral gel prior to centrifugation to separate agglutinated RRBCs from free RRBCs for visual examination. Following optimization of the gel card assays to distinguish between SARS-CoV-2-positive samples and negative controls, 10 clinical samples were tested in both gel cards and by indirect IgG ELISA. (
B) The results of indirect IgG ELISA comparing 10 samples, including PCR-confirmed SARS-CoV-2-positive samples and samples from healthy individuals collected before the SARS-CoV-2 outbreak. (
C) Digital images of gel card assays recorded from experiments could identify positive/negative of antibodies, negative control noted (“N”). Reprinted with permission from [
62].
ACS Sens. 2020, 5, 8, 2596–2603. Copyright 2020, American Chemical Society.
Figure 5. Ultra-rapid electrochemical immunosensor using aerosol jet nanoprinted reduced graphene oxide-coated 3D electrode for the detection of antibodies against SARS-CoV-2. (
A) Functionalization of the 3D electrode and sensing operation. (
a) AJ-printed gold micropillars prior to the surface treatment. (
b) Coating rGO sheets onto the electrodes. (
c) Immobilization of viral antigens onto rGO sheets. (
d) Selective binding of antibody with specific antigens after introduction. (
e) Schematics showing the sensing principle of the 3DcC device. (
f) Schematic illustration of the Nyquist plot alternation via electrical impedance spectroscopy (EIS) before and after antibody introduction and binding with the antigens on the electrode surface. (
B) The connection of the 3DcC device interfaced with a portable potentiostat to a smartphone via a USB-C connection for signal recording. (
C) Sensing performance of antibodies against SARS-CoV-2 spike S1 antigen at different molar concentrations from 1 fM to 20 nM in (
a) PBS solution and (
b) after sensor regeneration using low-pH chemistry. Reprinted with permission from [
65].