Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Air pollution has recently become a subject of increasing concern in many parts of the world. The World Health Organization (WHO) estimated that nearly 4.2 million early deaths are due to exposure to fine particles in polluted air, which causes multiple respiratory diseases. As a natural product, algae can be an alternative treatment due to potential biofunctional properties and advantages.
- algae
- particulate matter
- anti-inflammation
- respiratory diseases
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Particulate matter (PM) is a terminology describing a mixture of microscopic particles of solid and liquid matter found in the air [1]. Air pollution due to high levels of PM in the air is leading to various environmental issues and has global health implications, particularly in the area of public health. According to the World Health Organization (WHO), nearly 4.2 million early deaths are related to ambient air pollution [2]. Unfortunately, this emerging air pollution problem remains unsolved due to various reasons spanning from economical concern to geopolitical interest.
Generally, human activities such as open burning, emissions from factories and vehicles, rapid urbanization, development of the industrial sector, and coal-burning power plants are deemed as major causes of air pollution. In the South-East Asia (SEA) region, high concentrations of suspended PM are known to cause the haze phenomenon, which significantly affects health, crop production, the economy, and much more [3]. There are a variety of PM categories, of which some are based on aerodynamic diameter: coarse particles PM10 (2.5–10 µm), fine particles PM2.5 (<2.5 µm), and ultrafine particles PM0.1 (<0.1 µm or 100 nm); these are considered harmful pollutants. PM10 can deposit in the upper respiratory tract, whereas PM2.5 is primarily produced from the emission of combustion sources and can be more easily inhaled compared to PM10. They readily reach the alveoli of the lungs and affect the pulmonary and systematic circulation, while PM0.1 is primarily derived from vehicle emissions and can migrate from alveoli to the circulatory system [4].
Inhalation of high concentrations of particulate pollution may adversely impact human organs and body systems, especially the respiratory system, which might lead to different diseases, such as asthma, chronic obstructive pulmonary disease (COPD), lung cancer, and respiratory infections, and even combine with stroke and heart diseases [5]. As studied by Jiang, Mei, and Feng [6], individuals with asthma and COPD are vulnerable to the harmful consequence of air pollutants, which may lead to an increase in respiratory mortality and morbidity. Symptoms such as allergic reactions, wheezing, coughing, breathing problems, and inflammation responses in macrophage cells are found to be associated with exposure to air pollutants [7,8]. Hence, the inhibition of inflammation in the lungs is crucial. Therefore, there is an urgent need to search for effective and safe anti-inflammatory treatments that might prevent the relentless progression of respiratory diseases.
The search for novel treatments has led to the exploration of natural marine products as anti-inflammatory agents for treating inflammatory diseases such as respiratory diseases. Unlike terrestrial plants, algae have rapid growth rates and do not require arable land and freshwater for cultivation [9]. As algae sometimes grow in harsh conditions and are exposed to biotic and abiotic stresses, they possess unique metabolic pathways to produce secondary metabolites with biofunctional properties that might aid their survival [10]. Considering their advantages and therapeutic properties, algae have been a focus and actively explored for their potential nutraceuticals and pharmacological applications in recent years.
Previous studies reported that algae have anti-inflammatory properties due to the presence of bioactive metabolites [11,12,13,14]. However, there is a relatively low number of studies conducted specifically on anti-inflammatory activity against air pollutants, which is considered to be a unique area with great potential for application. Various seaweed-derived metabolites, such as fucosterol [15], alginic acid [16], dieckol [17], eckol [17], diphlorethohydroxycarmalol [18], gallic acid [19], mojabanchromanol [20], phlorotannins and flavonoid [21,22], have been proven to exhibit anti-inflammatory activity against air pollutants.
2. Pathways of Particulate Matter Travelling into the Respiratory System and Causing Inflammation
Ambient PM enters the respiratory system through inhalation and travels into the alveolar sacs to induce inflammation. PM such as PM2.5 contains polycyclic aromatic hydrocarbons (PAHs) that can stimulate inflammation in human bronchial epithelial cells [27]. When PAHs pass through the epithelium of alveoli, they can cause oxidative stress, activation of neutrophils, and mitochondrial damage. PM-induced oxidative stress may initiate intracellular ROS generation [28] and lipid peroxidation [29], whereby the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (NRf2/HO-1) pathway will be affected, resulting in the alteration of antioxidant enzyme transcription. The uncontrolled activation of polymorphonuclear neutrophils may increase the inflammatory responses, leading to tissue damage via oxidative stress and producing ROS [21]. It also enhances the inflammatory responses through the ROS-mediated activation of nuclear factor-kappa B (NF-κB) and MAPKs pathways.
The potential damage of mitochondria transmembrane caused by PM associated with ROS is another mechanism that may lead to mutagenicity and apoptosis. Mitochondria are the main target of ROS, and they lack nucleus and protective histones, which makes them specifically susceptible to ROS-stimulated destruction [30]. ROS can break the DNA strands when there is a failure of ROS catalyzation. This results in alterations in gene expression profiles and cellular biochemical profiles, leading to cell apoptosis, and remodeled cell function may be triggered by the responses of chromosome damage from PM stimulation [27]. This epigenetic and abnormal alteration could lead to lung cancer. On the other hand, lipid peroxidation can disrupt the double membrane of mitochondria and uncouple its membrane-bound complex [30]. When inflammation caused by oxidative stress is uncontrolled, PM can trigger inflammatory effects and cause changes in the lungs, associated with the elevated exacerbation of chronic respiratory conditions such as asthma and COPD (Figure 1).
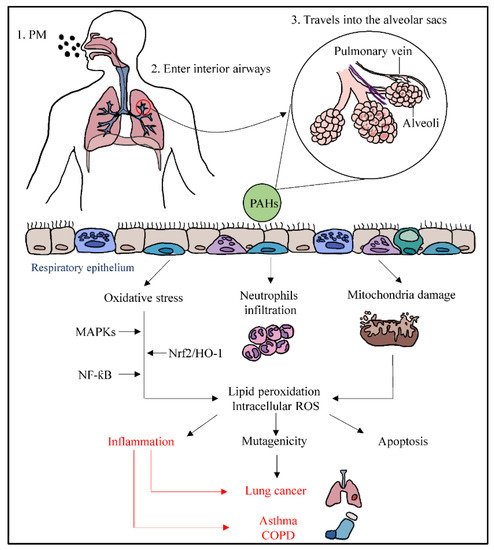
Figure 1. Pathways of particulate matter depositing into the respiratory system through inhalation, resulting in systemic lung inflammation, lung cancer, asthma, and COPD.
3. Algae Metabolites with Anti-Inflammatory Activity against Air Pollutants
According to the nine selected studies [7,15,16,17,18,19,20,21,22], only four brown algae, namely Ecklonia cava, Ishige okamurae, Sargassum binderi and Sargassum horneri from Korea, have been explored with respect to anti-inflammatory activity against air pollutants. The prominent secondary metabolite groups from these four brown algae with anti-inflammatory effects against PM are classified into phytosterol, polysaccharides, and polyphenols; furthermore, the ethanol extract complex of Sargassum horneri also exhibits similar anti-inflammatory effect. No studies have been reported on metabolites from red or green algae in this aspect. The chemical structures of some prominent metabolites are shown in Figure 2.

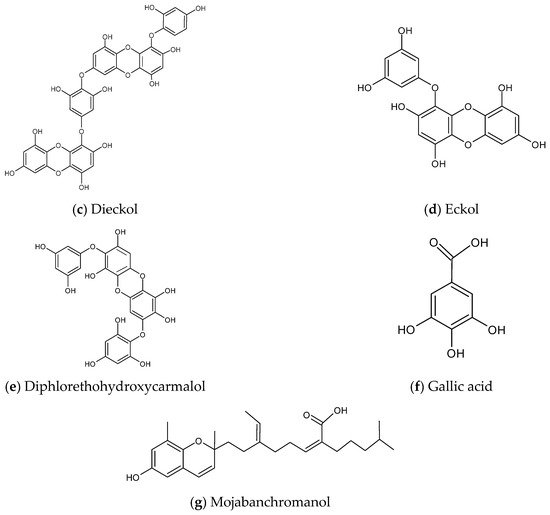
Figure 2. Chemical structures of metabolites derived from algae with anti-inflammatory effects against air pollutants. (a) Fucosterol, (b) Alginic acid, (c) Dieckol, (d) Eckol, (e) Diphlorethohydroxycarmalol, (f) Gallic acid, (g) Mojabanchromanol.
3.1. Phytosterols
Phytosterols are a group of the main compounds of lipids found in marine algae and have various beneficial health effects [31]. They are natural constituents of plant cell membranes with high similarity to cholesterol structure, but with minor differences in the position of ethyl and methyl groups [32]. Fucosterols are the most commonly found phytosterols in brown algae [33].
Fucosterol
Fucosterol from S. binderi is the most abundant phytosterol which has been proven to exhibit anti-inflammatory effects against damage induced by PM on the A549 immortalized alveolar basal epithelial cells [15]. It is mainly found to act through reducing the DNA damage on a cellular level, as observed from a decrease in the Sub-G1 cell population. As inflammation leads to oxidative stress-stimulated apoptosis, fucosterol significantly decreases the upregulation of inflammatory mediators (cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and inducible nitric oxide synthase (iNOS)) and pro-inflammatory cytokines (tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-beta (IL-1β)) expression by inhibiting the NF-κB and MAPKs pathways in a dose-dependent manner (12.5–50 µg/mL). This indicates that the expression levels of molecule mediators, such as IL-6 and TNF-α, could aid as a biomarker to assess the severity of fine dust (FD) exposure. FD consists of tiny particles with an aerodynamic diameter equal to or less than 10 µm [34]. The release of TNF-α and IL-6, the pro-inflammatory cytokines, increases chemotactic activity for neutrophils. Additionally, IL-1β is induced by PAH, and nickel in PM can further enhance mucin secretion, thicken airways, cause inflammation with macrophages and neutrophils, and develop symptoms into respiratory diseases such as COPD and asthma [19].
3.2. Polysaccharides
Polysaccharides are considered to be the largest group of compounds with biological activities in algae—approximately 60% of the metabolites [35]. They are water-soluble compounds with hydrophilic properties, and they have a regular structure that varies from linear to highly branched. They are formed by chains of monosaccharide units that connect via glycosidic bonds. Examples of the main compounds of polysaccharides include alginates, fucoidans, ulvans, carrageenans, and laminarans.
Alginic acid, a non-sulphated polysaccharide from brown alga S. horneri (SHA), when tested against the FD-stimulated inflammatory responses in HaCaT (human keratinocytes) and RAW264.7 mouse macrophages, showed anti-inflammatory effects against PM [16]. Evidently, the PAHs in PM and PM-derived ROS contribute to inflammation [27]. The upstream activations of NF-κB and MAPKs were measured to evaluate the inflammation caused by PAHs. SHA treatment had significantly increased the cell viability, and decreased the levels of pro-inflammatory cytokines, inflammatory mediators and intracellular ROS; inhibition of the NF-κB and MAPKs pathways was also observed.
3.3. Polyphenols
Polyphenolic compounds, also known as phenolics, are typically isolated from brown algae. They differ from simple molecules, including phenolic acids and other simple polyphenolic compounds, as well as more complex compounds such as phlorotannins, which are made up of phloroglucinol (1,3,5-trihydroxybenzene) units of polymeric structures [36]. Main compounds belonging to the group of polyphenolic compounds include eckols, fucols, fuhalols, phlorethols, fucophlorethols, bromophenols, terpenoids, phlorotannins and flavonoid.
Dieckol and Eckol
Sanjeewa et al. [17] proved that dieckol from brown alga Ecklonia cava showed anti-inflammatory potential against PM-induced damage on RAW264.7 macrophages. The dieckol effectively attenuated the levels of PGE2, NO, inflammatory mediators, and pro-inflammatory cytokines from the exposure of RAW264.7 to PM. Dieckol also protected the macrophages against cellular damage by decreasing the intracellular ROS through the activation of superoxide dismutase production, which leads to the Nrf2/HO-1 pathway. Nrf2/HO-1 are the essential antioxidant proteins that scavenge the production of NO through antioxidant mechanisms during the inflammation process [37]. Hence, dieckol can protect the cells against PM-stimulated inflammation and oxidative stress by inducing their anti-inflammatory and antioxidant pathways respectively.
Diphlorethohydroxycarmalol (DPHC)
Anti-inflammatory effects of DPHC from the brown alga I. okamurae in RAW264.7 macrophages, HaCaT cells and zebrafish embryos were investigated by Fernando et al. [18]. DPHC was proven to inhibit the production of PGE2, COX-2, IL-6 and IL-1β dose-dependently at concentrations of 6.25–25.00 µg/mL in HaCaT keratinocytes, and the expression of PGE2, NO, iNOS and TNF-α in macrophages. Furthermore, DPHC decreased the production of ROS and NO induced by FD in the zebrafish embryos, which consequently decreased cell death. Also, DPHC reduced the larval mortality and blocking of larval gills by FD. This indicates that the airway of the respiratory system (larval gills) of the in vivo experimental model will not be obstructed by FD after the DPHC treatment.
Phenolic Acid
Phenolic acid, particularly gallic acid from the brown alga S. horneri ethanol extract (SHE), showed anti-inflammatory effects against PM-induced type II alveolar epithelial cell lines, MLE-12 [19]. mRNA expression of toll-like receptors (TLRs) such as TLR2, TLR4 and TLR7, pro-inflammatory cytokines, and lung epithelial cell derived-chemokines (monocyte chemoattractant protein-1 (MCP-1), chemokine ligand 5 (CCL5) and IL-8) induced by PM were attenuated by the alga extract. The lung epithelial cell derived-chemokines are known to increase inflammatory responses, which can exacerbate asthmatic responses and lead to pulmonary sarcoidosis; thus, these chemokines should be reduced. mRNA expressions of IL-33 and pro-allergic cytokines thymic stromal lymphopoietin (TSLP) stimulated by PM were reduced as well. Additionally, the suppression of MAPK p38 phosphorylation by reducing tumor necrosis factor receptor-associated factor 6 (TRAF6) activation of proteins TNF and myeloid differentiation factor 88 (MyD88) was observed. PM-induced activation of the MAPK pathway in MLE-12 further suggested that it might be mediated through extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK).
Chromene (Mojabanchromanol)
Chromenes result from the dehydration of chromanols. They are phenolic terpenoids with a ring of aliphatic and chromanol side chains. Herath et al. [20] proved that mojabanchromanol (a novel chromene) in SHE exhibited anti-asthmatic effects in BALB/c mice. It has the potential in treating PM-induced allergic asthma, which is an inflammatory respiratory disease. Mitigation of PM-induced dendritic cells can be observed. PM-stimulated eosinophil infiltration, which is believed to be the origin of Th2-mediated asthma, attenuated in the trachea, lung and bronchoalveolar lavage fluid (BALF) due to SHE. As an elevation of infiltrated inflammatory cells in BALF is the dominant biomarker in asthma pathogenesis, it should be reduced [20]. Besides, stimulation of mast cells infiltration in response to the activation of Th2 cells was reduced in the allergic lung tissues. SHE also reversed the activation of mast cells which went through degranulation, and the release of histamine and vasoactive mediators in the trachea. As IgE is involved in mast cells activation and degranulation, analysis of serum IgE levels further proved the reduction level by SHE. Furthermore, airway obstruction and mucus released from goblet cells exacerbating asthma could be observed, but these were successfully attenuated by SHE.
Moreover, CD4+ and CD8+ T cell populations were investigated. Similarly, SHE suppressed the PM-induced T cell populations. As SHE reduced the CD4+ T cells, there was also decreased differentiation into Th2, whereas the reduced CD8+ T cells further attenuated the airway inflammation and hyperresponsiveness in PM-induced mice. Simultaneously, SHE further suppressed the Th17 cell response by reducing the mRNA expression of RAR-related orphan receptor gamma (RORγT) and phosphorylation of signal transducer and activator of transcription 3 (STAT3), which create the primary neutrophils for asthma. This led to the mitigation of neutrophil infiltration in the lung. mRNA expression of GATA3, the major transcription factor for Th2 cells differentiation, was also attenuated by SHE. PM-induced STAT5 translocation was another effect on transcription factors involved in the Th2 immune response, which was reduced by SHE. PM-stimulated IL-4, IL-5, IL-13 and IL-17a epithelial cell-derived cytokine expression was also reduced in asthmatic mice [20].
Phlorotannins and Flavonoid
The composition of metabolites such as phlorotannins and flavonoid from S. horneri ethanol extract (SHE) were proven to exhibit anti-inflammatory effects against PM in BALB/c mice models [21] and murine MH-S cells [22]. PM-induced NO secretion from the production of inflammatory mediators and pro-inflammatory cytokines was inhibited by phlorotannins and flavonoid from the SHE treatment. Besides, the metabolites from the SHE treatment-induced Nrf2 and HO-1 activities, resulting in the upregulation of antioxidant genes and cytoprotective potential against oxidative stress induced by FD in BALB/c mice [21]. Furthermore, MAPKs [21,22] and NF-κB [22] pathways were inhibited by the metabolites. Specifically, the upregulated mRNA expression levels of the TLRs in PM-stimulated MH-S cells were inhibited by the metabolites, leading to the downstream activations of both pathways [22].
3.4. Ethanol Extract
Besides bioactive compounds from algae, a relatively complex mixture derived from the solvent extraction method application on algae could also exhibit anti-inflammatory potential against the PM-induced damage. The protective effect of SHE against the FD-stimulated inflammation was investigated in RAW264.7 cells [7]. The production of inflammatory mediators and pro-inflammatory cytokines that stimulated NO production were inhibited by SHE treatment in the tested cells. Nrf2 and HO-1 activities were induced by SHE treatment, resulting in the upregulation of antioxidant genes and cytoprotective potential against oxidative stress induced by FD in RAW264.7. Furthermore, MAPKs pathways were attenuated by SHE.
4. Toxicity of Metabolites That Exhibit Anti-Inflammatory Effects
The toxicity of metabolites on experimental models against FD particles is usually examined by in vitro lactate dehydrogenase (LDH) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. The toxicity of algae metabolites tested was examined by dosing in a range of in vitro (e.g., A549 immortalized alveolar basal epithelial, RAW264.7 mouse macrophage, MLE-12 type II alveolar epithelial cell, and MH-S murine lung cells) cell lines and in vivo (e.g., zebrafish embryos, and BALB/c mice) experimental models. Fucosterol from S. binderi [15], DPHC from I. okamurae [18], dieckol and eckol from E. cava [17], phenolic acid from S. horneri [19], phlorotannins and flavonoid [21], and the ethanol extract of S. horneri [7] are largely non-toxic. Despite minor cytotoxic effects have been reported at concentrations of phenolic acid at ≥15.6 μg/mL [19]; DPHC at 50 µg/mL [18]; fucosterol at 100 µg/mL [15]; phlorotannins and flavonoid [21], ethanol extract [7], dieckol [17] and eckol [17] at 250 µg/mL on the tested experimental models, cytotoxicity was still well countered and more than 50% of cell viability could still be observed.
5. Mechanism of Actions of Algae to Inhibit Inflammation Caused by Particulate Matters
5.1. Inhibition of Mitogen-Activated Protein Kinase and Nuclear Factor-Kappa B
Stimulation of TLR2 and TLR4 can lead to inflammation by PM [38,39]. Metabolites from algae can inhibit the activation of TLRs, which are involved in inflammatory responses that stimulate the inflammatory signals through the downstream upregulation of TRAF6. Consequently, algae can suppress the MAPKs and NF-κB actions by stopping the upregulation of TRAF6 that promotes pro-inflammatory gene expression and transmits external inflammatory signals to the nucleus.
NF-κB represents heterodimers of both p50 and p65 subunits and locates in the cytoplasm as an inactive complex, binding to IκB-α [40]. The stimulation of the IκB-α kinase complex from LPS treatment can be inhibited by algae, resulting in the suppression of the triggered signaling pathway that also leads to the phosphorylation and activation of the MAPKs family. Algae can also inhibit the activated NF-κB, which acts as a transcription factor along with MAPKs, which trigger the COX-2, iNOS, and inflammatory cytokine production [21]. The initial degradation and ubiquitination of NF-κB, which occurs after translocation into the nucleus to initiate transcriptional processes, will be inhibited by algae treatment (Figure 3).
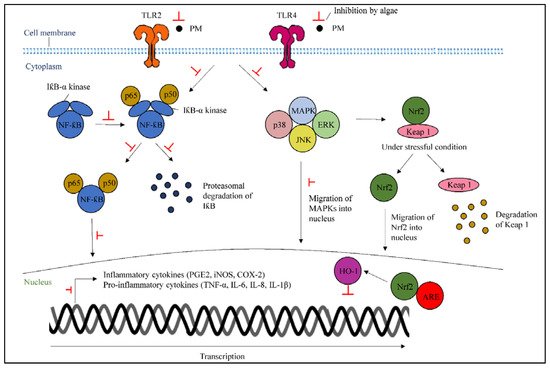
Figure 3. A scheme highlighting the particulate matter-stimulated inflammatory mechanisms, as well as indicating the underlying mechanisms of protective actions of algae-derived metabolites.
5.2. Activation of Nuclear Factor Erythroid 2-Related Factor 2/Heme Oxygenase-1
HO-1 is an anti-inflammatory enzyme that reduces inflammation and is regulated by Nrf2 in the inflammatory response. In the cytoplasm, Nrf2 is bound to the Keap1 protein, which is regulated through the MAPK pathway. Keap1 initiates subsequent Nrf2 degradation under normal conditions; however, the Nrf2/Keap1 molecule is separated under stressful conditions, resulting in the initiation of nucleus translocation and the attachment of Nrf2 to antioxidant response elements (ARE) in the promotors of specific genes in the nucleus [7]. Once the attachment is completed, the activation of HO-1 transcription can inhibit inflammatory responses. Hence, algae treatment could initiate the upregulation of Nrf2/HO-1 in the PM-induced cells (Figure 3).
This entry is adapted from the peer-reviewed paper 10.3390/md19060317
This entry is offline, you can click here to edit this entry!
