The antioxidant activity of anthocyanins in food is well known. Numerous antioxidant assays have been proposed to measure the capacity of anthocyanins to prevent the oxidation process that naturally occurs. Different solvents, temperatures, and pH levels are applied in each assay, and these factors should be taken into account in order to obtain useful and reproducible results. The concentration and the structure of these compounds are directly related to their antioxidant capacity and their environment. However, the effectiveness of the anthocyanin ingestion against diseases is also influenced by its bioavailability. Novel methodologies that simulate the digestion process have been developed in order to facilitate the current knowledge of anthocyanins bioavailability. Studies highlight the potential synergy effect between parent compounds and their derivatives (metabolites, conjugated products, and microbe-generated metabolites).
- anthocyanins
- antioxidant activity
- anthocyanin content
- bioavailability
- encapsulation
- therapeutic effects
1. Introduction
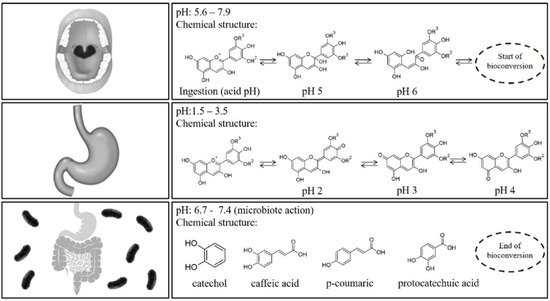
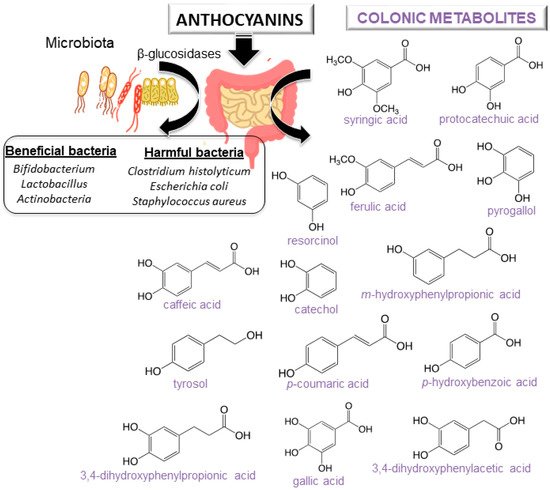
2. Bioavailability of Anthocyanins
3. Therapeutic Effects of Anthocyanins
| Eye Health | Administration | References |
|---|---|---|
| Improvement of vision in patients with open-angle glaucoma | Oral capsule | [56] |
| Protective effect during retinal inflammation | IV in rats | [57] |
| Regeneration of rhodopsin and smooth muscle relaxation | IV in mouse model | [58] |
| Improvement of dark adaptation | Oral capsule | [59] |
| Prevention of cataractogenesis of diabetic cataract | Incubation of Enucleated rat lenses | [60] |
| Antiapoptotic effects against oxidative damage of lens epithelial cell | Cell studies | [61] |
| Prevention of retinal degeneration induced by N-methyl-N-nitrosourea | Oral solution | [62] |
| Increase of ocular blood flows | Oral capsule | [63] |
| Cardiovascular diseases | ||
| Inhibition of platelet aggregation (in vitro antithrombotic properties) | Cell studies | [64] |
| Increase of high-density lipoprotein cholesterol levels and decrease of low-density lipoprotein cholesterol levels | Oral capsule | [65] |
| Lower risk of non-fatal myocardial infarction | Oral intake | [66] |
| Vasorelaxation properties in isolated coronary artery rings in pigs | Cell studies | [67] |
| Decrease of susceptibility to ischemia-reperfusion injury and infarct size | Rodent food | [68] |
| Improvement of lipid profile and platelet function | Oral capsule | [69] |
| Antiobesity effects | ||
| Improvement of weight gain and lipid profile on obese rats | Fat diet-induced mouse model | [70] |
| Suppression of body weight gain and improve blood lipid profile in rats | Fat diet-induced mouse model | [71] |
| Reduction of sugar concentration in urine and plasma in rats | Intraperitoneal and intragastric administration | [72] |
| Ameliorated obesity in high-fat-fed mice | Cell studies | [73] |
| Upregulation of adipocytokine secretion and gene expression in rat adipocytes | Cell studies | [74] |
| Suppression of fat tissue gain, weight gain and other metabolic disorders | Fat diet-induced mouse model | [75] |
| Antidiabetic effects | ||
| Amelioration of hyperglycemia and insulin sensitivity in diabetic mice | Fat diet-induced mouse model | [76] |
| Improvement of dyslipidemia, enhancement of antioxidant capacity, and prevention of insulin resistance in human with type 2 diabetes | Oral capsule | [77] |
| Alleviation of glomerular angiogenesis of diabetic kidneys in mice | Cell studies | [78] |
| Inhibition of DPP IV activity (a protease that regulates blood glucose levels via degradation of incretins) | Computational studies | [79] |
| Amelioration of renal apoptosis in diabetic nephropathy mice | Oral solution | [80] |
| Activation of adipose tissue-derived adiponectin to defend against diabetes-related endothelial dysfunction in mice | Diet-induced mouse model | [81] |
| Antimicrobial effects | ||
| Induction of cell damage by destroying the cell wall, membrane, and intercellular matrix | Cell studies | [82] |
| Highest sensitivity to Aeromonas hydrophila and Listeria innocua | Microbial strains | [83] |
| Antibacterial effects towards Enterococcus faecium resistant to vancomycin, Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli | Microbial strains | [84] |
| Inhibition of Gram-negative bacteria | Microbial strains | [85] |
| Anticancer effects | ||
| Suppression of cell proliferation, inflammation, and angiogenesis and induction of apoptosis in esophageal tissue of rats | Diet-induced rat model | [86] |
| Anti-invasive potential in breast cancer cell lines | Cell studies | [87] |
| Anticancer effect on BALB/c nude mice bearing MDA-MB-453 cell xenografts and breast cancer cell lines | Cell studies | [88] |
| Inhibition of cell migration and invasion, suppression of activation of rapidly accelerated fibrosarcoma, mitogen-activated protein kinase and c-Jun N-terminal kinase, and downregulation of secretion of matrix metalloproteinase 2 | Cell studies | [89] |
| Inhibition of growth of human HT-29 colon cancer cells, increase of expression of tumor suppression genes and decrease of cyclooxygenase-2 gene expression | Cell studies | [90] |
| Reduction of colonic aberrant crypt foci, colonic cellular proliferation and COX-2 mRNA expression in rats | Diet-induced rat model | [91] |
| Suppression of formation of aberrant crypt foci in colons of CF-1 mice | Cell studies and diet-induced rat model | [92] |
| Promotion of apoptosis in benign prostatic hyperplasia rats | Oral doses in rat model | [93] |
| Anti-invasive effect on human hepatoma Hep3B cells and inhibition of matrix metalloproteinase MMP-2 and MMP-9 gene expression | Cell studies | [94] |
| Inhibition of Akt-mTOR signaling thereby inducing maturation of acute myeloid leukemia cells, besides inducing apoptotic players such as TRAIL in cancer systems | Cell studies | [95] |
| Neurodegenerative diseases | ||
| Neuroprotective activity by suppression of dopaminergic cell death in Parkinson’s disease | Cell studies | [96] |
| Improvement of learning and memory ability in mice. Higher antioxidant enzyme activity and less lipid oxidation in both brain and liver | Diet-induced mouse model | [97] |
| Regulation of cholinergic neurotransmission to restore Na+, K+-ATPase and Ca2+-ATPase activities and to prevent memory deficits in rats | Oral and injected rat models | [98] |
| Neuroprotective effect: Memory and synaptic dysfunction | Oral rat models | [99] |
| Improvement of its free radical scavenging capabilities via p38/JNK pathway against Abeta1-42-induced oxidative stress | Cell studies | [100] |
| Enhancement of neuroprotection against Abeta1-42-induced neuroinflammation and neurodegeneration | Oral mouse model and cell studies | [101] |
| Enhancement of the neuroprotection in an Abeta1-42 mouse model of Alzheimer’s disease | Oral mouse model and cell studies | [102] |
This entry is adapted from the peer-reviewed paper 10.3390/antiox9050451
References
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970.
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15.
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180.
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Technol. 2016, 7, 375–393.
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520.
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238.
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant Capacity of Anthocyanin Pigments. In Flavonoids—From Biosynthesis to Human Health; Justino, J., Ed.; Science, Technology and Medicine Open Access Publisher: Rijeka, Croatia, 2017; Chapter 11; pp. 205–255.
- Martín Bueno, J.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jímenez, A.M.; Fett, R.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151.
- Navas, M.J.; Jiménez-Moreno, A.M.; Martín Bueno, J.; Sáez-Plaza, P.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part IV: Extraction of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342.
- Gamel, T.H.; Wright, A.J.; Tucker, A.J.; Pickard, M.; Rabalski, I.; Podgorski, M.; Di Ilio, N.; O’Brien, C.; Abdel-Aal, E.M. Absorption and metabolites of anthocyanins and phenolic acids after consumption of purple wheat crackers and bars by healthy adults. J. Cereal Sci. 2019, 86, 60–68.
- Cavalcante Braga, A.R.; Murador, D.C.; Mendes de Souza Mesquita, L.; Vera de Rosso, V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40.
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66.
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919.
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brncˇic, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2020, 9, 2.
- Mülleder, U.; Murkovic, M.; Pfannhauser, W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods 2002, 53, 61–66.
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242.
- Felgines, C.; Talavéra, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.-L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727.
- Passamonti, S.; Vanzo, A.; Vrhovsek, U.; Terdoslavich, M.; Cocolo, A.; Decorti, G.; Mattivi, F. Hepatic uptake of grape anthocyanins and the role of bilitranslocase. Food Res. Int. 2005, 38, 953–960.
- Sandoval-Ramírez, B.A.; Catalanń, U.; Fernanńdez-Castillejo, S.; Rubió, L.; Maciá, A.; Sola, R. Anthocyanin tissue bioavailability in animals: Possible implications for human health. A systematic review. J. Agric. Food Chem. 2018, 66, 11531–11543.
- Lucioli, S. Anthocyanins: Mechanism of action and therapeutic efficacy. In Medicinal Plants as Antioxidant Agents: Understanding Their Mechanism of Action and Therapeutic Efficacy; Capasso, A., Ed.; Research Signpost: Kerala, India, 2012; pp. 27–57.
- Jin, Y.A.D.; George, T.G.M.; Lovegrove, J.A.A. Randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Clin. Nutr. 2011, 65, 849–857.
- Wu, X.; Cao, G.; Prior, R.L. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J. Nutr. 2002, 132, 1865–1871.
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104 (Suppl. S3), S48–S66.
- Kaiser, M.; Müller-Ehl, L.; Passon, M.; Schieber, A. Development and validation of methods for the determination of anthocyanins in physiological fluids via UHPLC-MSn. Molecules 2020, 25, 518.
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769.
- Ferrars, R.M.; Cassidy, A.; Curtis, P.; Kay, C.D. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol. Nutr. Food Res. 2014, 58, 490–502.
- Ferrars, R.M.; Czank, C.; Saha, S.; Needs, P.W.; Zhang, Q.; Raheem, K.S.; Botting, N.P.; Kroon, P.A.; Kay, C.D. Methods for isolating, identifying, and quantifying anthocyanin metabolites in clinical samples. Anal. Chem. 2014, 86, 10052–10058.
- Nurmi, T.; Mursu, J.; Heinonen, M.; Nurmi, A.; Hiltunen, R.; Voutilainen, S. Metabolism of berry anthocyanins to phenolic acids in humans. J. Agric. Food Chem. 2009, 57, 2274–2281.
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003.
- Novotny, J.A. Anthocyanin Bioavailability: Past Progress and Current Challenges. In Emerging Trends in Dietary Components for Preventing and Combating Disease; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Chapter 32; pp. 559–568.
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus Microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449.
- Gowd, V.; Karim, N.; Xie, L.; Shishir, M.R.I.; Xu, Y.; Chen, W. In vitro study of bioaccessibility, antioxidant, and α-glucosidase inhibitory effect of pelargonidin-3-O-glucoside after interacting with beta-lactoglobulin and chitosan/pectin. Int. J. Biol. Macromol. 2020, 154, 380–389.
- Gowd, V.; Bao, T.; Chen, W. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Res. Int. 2019, 120, 523–533.
- Zhou, L.; Xiec, M.; Yanga, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. Food Sci. Technol. 2020, 117, 628101.
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, 0195754.
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890.
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938.
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. Peer J. 2018, 2018, 1–31.
- Lacombe, A.; Li, R.W.; Klimis-Zacas, D.; Kristo, A.S.; Tadepalli, S.; Krauss, E.; Young, R.; Wu, V.C.H. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS ONE 2013, 8, 67497.
- Flores, G.; Ruiz del Castillo, M.L.; Costabile, A.; Klee, A.; Bigetti Guergoletto, K.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods 2015, 16, 50–57.
- Guergoletto, K.B.; Costabile, A.; Flores, G.; Garcia, S.; Gibson, G.R. In vitro fermentation of juçara pulp (Euterpe edulis) by human colonic microbiota. Food Chem. 2016, 196, 251–258.
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098.
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077.
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230.
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Dry Technol. 2014, 32, 509–518.
- Robert, P.; Fredes, C. The encapsulation of anthocyanins from berry-type fruits. Trends in foods. Molecules 2015, 20, 5875–5888.
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509.
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523.
- Fernandes, A.; Rocha, M.; Santos, L.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastro- intestinal stabilization. Food Chem. 2018, 245, 426–431.
- Huang, Y.; Zhou, W.B. Microencapsulation of anthocyanins through two-step emulsification and release characteristics during in vitro digestion. Food Chem. 2019, 278, 357–363.
- Khoo, H.E.; Lim, S.M.; Azlan, A. Evidence-based therapeutic effects of anthocyanins from foods. Pak. J. Nutr. 2019, 18, 1–11.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299.
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary sources. J. Sci. Food Agric. 2000, 80, 1063–1072.
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food 2012, 15, 818–823.
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235.
- Miyake, S.; Takahashi, N.; Sasaki, M.; Takahashi, N.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin- rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012, 92, 102–109.
- Lee, J.; Lee, H.K.; Kim, C.Y.; Hong, Y.J.; Choe, C.M.; You, T.W.; Seong, G.J. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. Br. J. Nutr. 2005, 93, 895–899.
- Thiraphatthanavong, P.; Wattanathorn, J.; Muchimapura, S.; Wipawee, T.M.; Wannanon, P.; Terdthai, T.U.; Suriharn, B.; Lertrat, K. Preventive effect of Zea mays L. (purple waxy corn) on experimental diabetic cataract. BioMed Res. Int. 2014, 2014, 507435.
- Mok, J.W.; Chang, D.J.; Joo, C.K. Antiapoptotic effects of anthocyanin from the seed coat of black soybean against oxidative damage of human lens epithelial cell induced by H2O2. Curr. Eye Res. 2014, 39, 1090–1098.
- Paik, S.S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.H.; Kim, I.B. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Exp. Eye Res. 2012, 97, 55–62.
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica 2012, 228, 26–35.
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, W.; Li, C.; Zhu, G.; Wang, Y.; Freedman, J.J.; Ling, W.; Ni, H. Anthocyanins inhibit platelet activation and attenuate thrombus growth in both human and murine thrombosis models. Blood 2010, 116, 3197.
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492.
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594.
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170.
- Toufektsian, M.C.; De Lorgeril, M.; Nagy, N.; Salen, P.; Donati, M.B.; Giordano, L.; Mock, H.-P.; Peterek, S.; Matros, A.; Petroni., K.; et al. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J. Nutr. 2008, 138, 747–752.
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; di Stefano, G. One month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294.
- Kwon, S.H.; Ahn, I.S.; Kim, S.O.; Kong, C.S.; Chung, H.Y.; Do, M.S.; Park, K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food 2007, 10, 552–556.
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661.
- Jankowski, A.; Jankowska, B.; Niedworok, J. The effects of anthocyanin dye from grapes on experimental diabetes. Folia Med. Cracov. 2000, 41, 5–15.
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31.
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157.
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3- O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130.
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533.
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748.
- Kang, M.K.; Lim, S.S.; Lee, J.Y.; Yeo, K.M.; Kang, Y.-H. Anthocyanin-rich purple corn extract inhibit diabetes-associated glomerular angiogenesis. PLoS ONE 2013, 8, 79823.
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and citrus phenolic compounds inhibit dipeptidyl peptidase IV: Implications in diabetes management. Evid. Based Complement. Alternat. Med. 2013, 2013, 479505.
- Koh, E.S.; Lim, J.H.; Kim, M.Y.; Chung, S.; Shin, S.J.; Choi, B.S.; Kim, H.W.; Hwang, S.Y.; Kim, S.W.; Park, C.W.; et al. Anthocyanin-rich Seoritae extract ameliorates renal lipotoxicity via activation of AMP-activated protein kinase in diabetic mice. J. Transl. Med. 2015, 13, 203.
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E975–E988.
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508.
- Genskowsky, E.; Puente, L.A.; Perez-Alvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agr. 2016, 96, 4235–4242.
- Côté, J.; Caillet, S.; Doyon, G.; Ng, K. Antimicrobial effect of cranberry juice and extracts. Food Cont. 2011, 22, 1413–1418.
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507.
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. 2009, 2, 84–93.
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother. Res. 2010, 24, 1862–1869.
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136.
- Chen, X.Y.; Zhou, J.; Luo, L.P.; Han, B.; Li, F.; Chen, J.Y.; Zhu, Y.F.; Chen, W.; Yu, X.P. Black rice anthocyanins suppress metastasis of breast cancer cells by targeting RAS/RAF/MAPK pathway. BioMed Res. Int. 2015, 2015, 414250.
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196.
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93.
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917.
- Jang, H.; Ha, U.S.; Kim, S.J.; Yoon, B.I.; Han, D.S.; Yuk, S.M.; Kim, S.W. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J. Agric. Food Chem. 2010, 58, 12686–12691.
- Shin, D.Y.; Lee, W.S.; Kim, S.H.; Kim, M.J.; Yun, J.W.; Lu, J.N.; Lee, S.J.; Tsoy, I.; Kim, H.J.; Ryu, C.H.; et al. Anti-invasive activity of anthocyanins isolated from Vitis coignetiae in human hepatocarcinoma cells. J. Med. Food. 2009, 12, 967–972.
- Bontempo, P.; de Masi, L.; Carafa, V.; Rigano, D.; Scisciola, L.; Iside, C.; Grassi, R.; Molinari, A.M.; Aversano, R.; Nebbioso, A.; et al. Anticancer activities of anthocyanin extract from genotyped Solanum tuberosum L. “Vitelotte”. J. Funct. Foods 2015, 19, 584–593.
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective effects of anthocyanin and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 1555, 60–77.
- Shih, P.-H.; Chan, Y.-C.; Liao, J.-W.; Wang, M.-F.; Yen, G.-C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605.
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.C.; Agostinho, P.; Marisco, P.C. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int. J. Dev. Neurosci. 2014, 33, 88–97.
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 2017, 54, 255–271.
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Abeta1-42-induced oxidative stress. J. Nanobiotechnol. 2017, 15, 12.
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Abeta1-42-induced neuroinflammation and neurodegeneration via the NF-KB /JNK/GSK3beta signaling pathway. Nanomedicine 2017, 13, 2533–2544.
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold nanoparticles enhanced the neuroprotection of anthocyanins in an Abeta1-42 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017, 54, 6490–6506.
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24, 3311.
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asian Pac. J. Clin. Nutr. 2007, 16, 200–208.
- Tsuda, T. Anthocyanins as functional food factors: Chemistry, nutrition and health promotion. Food Sci. Technol. Res. 2012, 18, 315–324.
- Smeriglio, A.; Monteleone, D.; Trombetta, D. Health effects of Vaccinium myrtillus L.: Evaluation of efficacy and technological strategies for preservation of active ingredients. Mini Rev. Med. Chem. 2014, 14, 567–584.
- Liu, L.K.; Lee, H.J.; Shih, Y.W.; Chyau, C.C.; Wang, C.J. Mulberry anthocyanins extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J. Food Sci. 2008, 73, 4113–4121.
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334.
- Luís, Â.; Domingues, F.; Pereira, L. Association between berries intake and cardiovascular diseases risk factors: A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018, 9, 740–757.
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andresń-Lacueva, C.; dePascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.J.; et al. Meta-Analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694.
- Ksonzékova, P.; Mariychuk, R.; Eliasova, A.; Mudronova, D.; Csank, T.; Kiraly, J.; Marcincakova, D.; Pistl, J.; Tkacikova, L. In vitro studies of biological activities of anthocyanin-rich berry extracts on porcine intestinal epithelial cells. J. Sci. Food Agric. 2016, 96, 1093–1100.
- Bone, K.; Mills, S. Principles and Practice of Phytotherapy: Modern Herbal Medicine; Churchill Livingstone: Edinburg, TX, USA, 2013.
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24.
- Rupasinghe, H.P.V.; Arumuggam, N. Health benefits of anthocyanins. Food Chem. Func. Anal. 2019, 2019, 123–158.
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of beneficial metabolic effects of berries in high-fat fed C57BL/6J mice. J. Nutr. Metab. 2014, 2014, 403041.
- Lachin, T.; Reza, H. Anti diabetic effect of cherries in alloxan induced diabetic rats. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 67–72.
- Orqueda, M.E.; Torres, S.; Zampini, I.C.; Cattaneo, F.; Fernandez Di Pardo, A.; Valle, E.M.; Jimenez-Aspee, F.; Schmeda-Hirschmann, G.; Isla, M.I. Integral use of Argentinean Solanum betaceum red fruits as functional food ingredient to prevent metabolic syndrome: Effect of in vitro simulated gastroduodenal digestión. Heliyon 2020, 6, 03387.
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933.
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156.
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595.
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Anthocyanins and Human Health: Biomolecular and Therapeutical Aspects; Springer: New York, NY, USA, 2016.
- Wallace, T.C.; Giusti, M.M. Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2014.
- Fraga, C.G. (Ed.) Plant Phenolics and Human Health: Biochemistry, Nutrition and Pharmacology; Wiley: New York, NY, USA, 2010.
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187.
- Galvano, F.; Salamone, F.; Nicolosi, A.; Vitaglione, P. Anthocyanins-based drugs for colon cancer treatment: The nutriotionist’s point of view. Cancer Chemother. Pharmacol. 2009, 64, 431–432.
- Webb, M.R.; Min, K.; Ebeler, S.E. Anthocyanins interactions with DNA: Intercalation, topoisomerase I inhibition and oxidative reactions. J. Food Biochem. 2008, 32, 176–196.
- Fernandes, I.; Faria, A.; Azevedo, J.; Soares, S.; Calhau, C.; De Freitas, V.; Mateus, N. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J. Agric. Food Chem. 2010, 58, 3785–3792.
- Sun, J.; Hai Liu, R. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006, 241, 124–134.
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology 2015, 68, 1207–1213.
- Im, N.K.; Jang, W.J.; Jeong, C.H.; Jeong, G.S. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-kappaB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food. 2014, 17, 855–861.
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6- trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 2014, 53, 432–439.
- Chen, P.N.; Kuo, W.H.; Chiang, C.L.; Chiou, H.L.; Hsieh, Y.S.; Chu, S.C. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem. Biol. Interact. 2006, 163, 218–229.
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741.
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure–function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398.
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128.
- Winter, A.N.; Bickford, P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333.
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350.
