Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Salinity is an issue impairing crop production across the globe. Under salinity stress, besides the osmotic stress and Na+ toxicity, ROS (reactive oxygen species) overaccumulation is a secondary stress which further impairs plant performance. Chloroplasts, mitochondria, the apoplast, and peroxisomes are the main ROS generation sites in salt-stressed plants.
- ROS Scavenging
- antioxidant enzymes
- non-enzymatic scavenging system
1. Enzymatic Scavenging System
Protection of chloroplasts from ROS accumulation is very important for stress tolerance in plants. Superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR) constitute an efficient ascorbate–glutathione cycle (ASA–GSH, Figure 1), which is a part of the enzymatic antioxidant system of plants [1][2][3][4][5]. In chloroplasts, a major ROS-scavenging pathway in plants is the water–water cycle (WWC). WWC can protect enzymes in chloroplasts and PSI from ROS and dissipate excess light energy in plant cells [6].
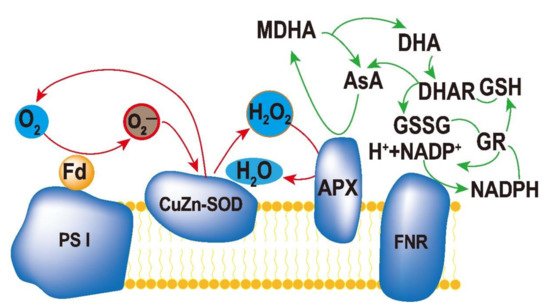
Figure 1. Ascorbate–glutathione cycle in plant cells. The reduced form of ascorbate (Asc) is oxidized to monodehydroascorbate (MDHA), which can either be reduced by monodehydroascorbate reductase (MDHAR) to Asc, or react to dehydroascorbate (DHA). DHA can be reduced by dehydroascorbate reductase (DHAR) to Asc. The reduced form of glutathione (GSH) is oxidized to glutathione disulfide (GSSG), which is reduced by glutathione reductase (GR) to GSH. During the reduction of MDHA and GSSG, the electron acceptor NADP is regenerated.
-
(1) 2H2O → 4[e−] + 4H+ + O2 (The photolysis of H2O in PSII);
-
(2) 2O2 + 2[e−] → 2 O2− (Photoreduction of O2 in PSI);
-
(3) 2O2− + 2H+ → H2O2 + O2 (SOD catalyzes O2− disproportionation);
-
(4) H2O2 + 2AsA → 2H2O + 2MDA (APX catalyzes ASA reduction H2O2);
-
(5) 2MDA + 2[e−] + 2H+ → 2AsA.
-
Total: 2H2O + O2− → O2 + 2H2O.
A previous study indicated that optimum exogenous selenium enhanced the activity of POD and APX, which ultimately protected chloroplasts from auto-pepsia and resulted in a higher photosynthesis rate in maize crop grown under salt stress [7]. Transcriptomic and metabolomic analyses revealed that genes encoding LEA (late-embryogenesis-abundant) proteins are up-regulated under salt stress. It is evident that LEA proteins could enhance the antioxidant enzyme activities [8]. It is evident that halophytes have a stronger enzymatic antioxidant system (CAT and SOD), and this capability has been used as a selection criterion for salt-tolerant varieties [9][10][11]. A previous study reported that SOD converted salt-induced ROS into H2O2, and APX maintained H2O2 at an optimum level as a signal molecule [12]. The study also revealed the importance of PaSOD (superoxide dismutase) and RaAPX (ascorbate peroxidase) gene in plant salt tolerance, which could enhance the biosynthesis of compatible solutes and lignin. Overexpression of mitochondria-located protein 4-hydroxybenzoate polyprenyl diphosphate transferase (PPT) increased the ubiquinone content and enhanced the plant’s salt-tolerance. Glutathione peroxidase (GPX) is an important component of the ASA–GSH cycle. The ASC (ascorbate)/GSH cycle is also an important antioxidant system in plants. A study on the glycophyte Brassica juncea and the halophyte Sesuvium portulacastrum indicated that the ASC/DHA (dehydroascorbate) and GSH/GSSG (glutathione disulfide) ratios were maintained in B. juncea but increased in S. portulacastrum [11]. By using OsGPX3-RNAi silenced plants, researchers found that GPX had an important role in plant CO2 assimilation, biomass accumulation, and PS II protection under salt stress [13]. Interestingly, OsGPX3-RNAi silenced plants displayed no observable difference with untransformed plants in ROS accumulation, suggesting a complex role for GPX in plant response to salt tolerance [14]. In addition, the gene related to alternative oxidase (AOX) located in the inner membrane of mitochondria has been reported, and is believed to be involved in the response to abiotic stress [15].
2. Non-Enzymatic Scavenging System
Several highly toxic ROS, such as 1O2 and •OH, cannot be scavenged by enzymatic antioxidant systems. Thus, plants depend solely on non-enzymatic means to scavenge those ROS. Generally, ROS lead to lipid peroxidation [16][17] and an increase in monodehydroascorbate (MDA) content [18]. Overexpression of IbWRKY2 induced MDA accumulation in plant cells, coupled with ROS-scavenging genes up-regulation, resulting in improved salt tolerance [19]. Carotenoids and α-tocopherol, as components of non-enzymatic antioxidant systems, can also scavenge ROS. For example, carotenoids are distributed in the thylakoid pigment—protein complexes in close proximity to chlorophylls near the potential sites of 1O2 formation. Furthermore, melatonin is considered a small molecule that may scavenge ROS, and the “manager” in plants under salinity stress. For example, a previous study found that the gene related to the melatonin synthesis enzyme N-acetylserotonin O-methyltransferase (MzASMT9), which is localized in the chloroplast, is upregulated by salt stress [20]. Additionally, MTs (metallothioneins) interact with AtVDAC3 (mitochondrial membrane voltage-dependent anion channels) and regulate ROS homeostasis [21]. Flavodoxin is known as an electron transfer shuttle, and can drive reducing equivalents away from oxygen, preventing ROS generation [22]. However, interestingly, compared with the enhanced tolerance to drought, excess irradiation, temperature stress, and iron starvation, overexpression of the plastid-targeted cyanobacterial flavodoxin in tobacco plants failed to result in increased salt resistance [23].
This entry is adapted from the peer-reviewed paper 10.3390/su13063552
References
- Loggini, B.; Scartazza, A.; Brugnoli, E.; Navari-Izzo, F. Antioxidative Defense System, Pigment Composition, and Photosynthetic Efficiency in Two Wheat Cultivars Subjected to Drought. Plant Physiol. 1999, 119, 3–1091.
- Hasanuzzaman, M.; Borhannuddin, B.M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2020, 8, 681195–681203.
- Sarker, U.; Oba, S. Catalase, Superoxide Dismutase and Ascorbate-Glutathione Cycle Enzymes Confer Drought Tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496.
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410.
- Diaz-Vivancos, P.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic Expression of Cytosolic Superoxide Dismutase and Ascorbate Peroxidase Leads to Salt Stress Tolerance in Transgenic Plums. Plant Biotech. J. 2013, 11, 976–985.
- Prihoda, J.; Tanaka, A.; de Paula, W.B.M.; Allen, J.F.; Tirichine, L.; Bowler, C. Chloroplast-Mitochondria Cross-Talk in Diatoms. J. Exp. Bot. 2012, 63, 1543–1557.
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of Exogenous Selenium Supply on Photosynthesis, Na+ Accumulation and Antioxidative Capacity of Maize (Zea mays L.) under Salinity Stress. Sci. Rep. 2017, 7, 42039.
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of The Late Embryogenesis Abundant (Lea) Proteins Family and Their Role in Drought Stress Tolerance in Upland Cotton. BMC Genet. 2018, 19, 1–31.
- Prashanth, S.R.; Sadhasivam, V.; Parida, A. Over Expression of Cytosolic Copper/Zinc Superoxide Dismutase from A Mangrove Plant Avicennia Marina in Indica Rice Var Pusa Basmati-1 Confers Abiotic Stress Tolerance. Transgenic Res. 2008, 17, 281–291.
- Yıldıztugay, E.; Sekmen, A.H.; Turkan, I.; Kucukoduk, M. Elucidation of Physiological and Biochemical Mechanisms of an Endemic Halophyte Centaurea tuzgoluensis under Salt Stress. Plant Physiol. Biochem. 2011, 49, 816–824.
- Srivastava, A.K.; Esrivastava, S.; Lokhande, V.H.; D’souza, S.F.; Esuprasanna, P. Salt Stress Reveals Differential Antioxidant and Energetics Responses in Glycophyte (Brassica Juncea L.) and Halophyte (Sesuvium Portulacastrum L.). Front. Environ. Sci. 2015, 3.
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX Genes Positively Regulates Secondary Cell Wall Biosynthesis and Promotes Plant Growth and Yield in Arabidopsis Under Salt Stress. Plant Mol. Biol. 2015, 87, 615–631.
- Paiva, A.L.S.; Passaia, G.; Lobo, A.K.M.; Jardim-Messeder, D.; Silveira, J.A.; Margis-Pinheiro, M. Mitochondrial Glutathione Peroxidase (Osgpx3) Has A Crucial Role in Rice Protection Against Salt Stress. Environ. Exp. Bot. 2019, 158, 12–21.
- Zhang, Y.; Zhao, H.; Zhou, S.; He, Y.; Luo, Q.; Zhang, F.; Qiu, D.; Feng, J.; Wei, Q.; Chen, L.; et al. Expression of Tagf14b, A 14-3-3 Adaptor Protein Gene from Wheat, Enhances Drought and Salt Tolerance in Transgenic Tobacco. Planta 2018, 248, 117–137.
- Borecký, J.; Nogueira, F.T.S.; De Oliveira, K.A.P.; Maia, I.G.; Vercesi, A.E.; Arruda, P. The Plant Energy-Dissipating Mitochondrial Systems: Depicting the Genomic Structure and The Expression Profiles of The Gene Families of Uncoupling Protein and Alternative Oxidase in Monocots and Dicots. J. Exp. Bot. 2006, 57, 849–864.
- Garg, S.K.; Tripathi, M.; Singh, S.K.; Singh, A. Pentachlorophenol Dechlorination and Simultaneous Cr6+ Reduction by Pseudomonas Putida SKG-1 MTCC (10510): Characterization of PCP Dechlorination Products, Bacterial Structure, and Functional Groups. Environ. Sci. Pollut. Res. 2012, 20, 2288–2304.
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53.
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250.
- Zhu, H.; Zhou, Y.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. A Novel Sweetpotato Wrky Transcription Factor, Ibwrky2, Positively Regulates Drought and Salt Tolerance in Transgenic Arabidopsis. Biomolecules 2020, 10, 506.
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J. Chloroplastic Biosynthesis of Melatonin and its Involvement in Protection of Plants from Salt Stress. Sci. Rep. 2017, 7, 41236.
- Gao, Y.; Ma, J.; Zheng, J.; Chen, J.; Chen, M.; Zhou, Y.; Fu, J.; Xu, Z.; Ma, Y. The Elongation Factor GmEF4 is Involved in the Response to Drought and Salt Tolerance in Soybean. Inter. J. Mol. Sci. 2019, 20, 3001.
- Zurbriggen, M.D.; Tognetti, V.B.; Fillat, M.F.; Hajirezaei, M.-R.; Valle, E.M.; Carrillo, N. Combating Stress with Flavodoxin: A Promising Route for Crop Improvement. Trends Biotechnol. 2008, 26, 531–537.
- Fisher, B.; Yarmolinsky, D.; Abdel-Ghany, S.; Pilon, M.; Pilon-Smits, E.A.; Sagi, M.; van Hoewyk, D. Superoxide Generated from the Glutathione-mediated Reduction of Selenite Damages the Iron-sulfur Cluster of Chloroplastic Ferredoxin. Plant Physiol. Biochem. 2016, 106, 228–235.
This entry is offline, you can click here to edit this entry!
