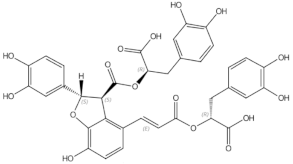
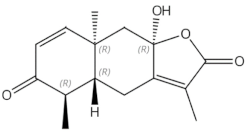
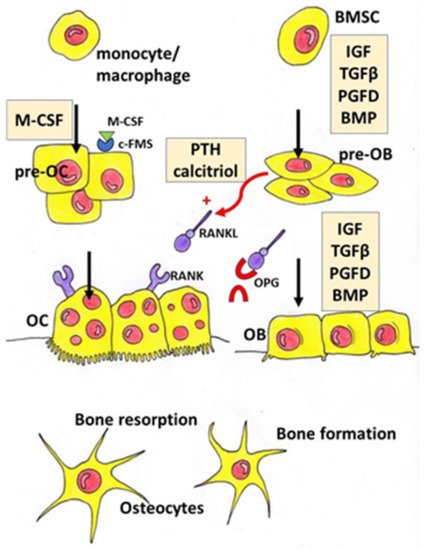
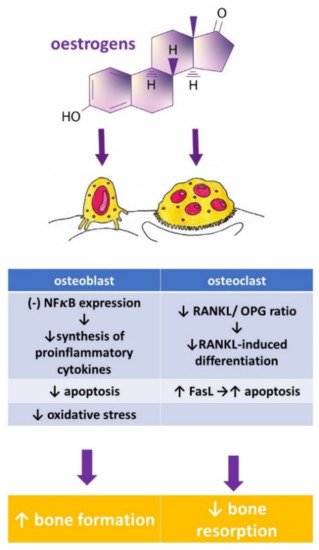
Osteoporosis is a systemic bone disease characterized by reduced bone mass and the deterioration of bone microarchitecture leading to bone fragility and an increased risk of fractures. Conventional anti-osteoporotic pharmaceutics are effective in the treatment and prophylaxis of osteoporosis, however they are associated with various side effects that push many women into seeking botanicals as an alternative therapy.
- osteoporosis
- menopause
- botanicals
- herbs
1. Introduction
|
Herbal Compounds |
Subgroup |
Chemical Structure |
Proposed Mechanism of Action |
|---|---|---|---|
|
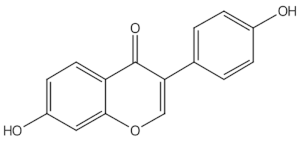
Daidzein |
isoflavones |
|
ER mediated signalling pathway, activation of intracellular pathways: AKT, phospholipase C (PLC), mitogen-activated protein kinase (MAPK) [14] |
|
Genistein |
isoflavones |
|
ER-mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
|
Ipriflavone |
isoflavones |
|
Modulation of key signalling pathways to regulate bone resorption (e.g., ↓urinary DPD, NTX) and bone formation (e.g., ↑BALP and osteocalcin [15] |
|
Biochanin A |
O-methylated isoflavones |
|
ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
|
Formononetin |
O-methylated isoflavones |
|
ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
|
Glycitein |
O-methylated isoflavones |
|
ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
|
Icariin |
prenylated flavonol glycoside |
|
Stimulation of bone formation by promotion of osteoblasts differentiation and enhancement of their activity [16]; activation of BMP-2/Smad4, Wnt and IGF-1 signal transduction pathways [5,17], induction of ERK, JNK and p38 kinase activation [18]; decreasing of RANKL-induced osteoclastogenesis via inhibition of NFκB and MAPK expression [19] |
|
8-prenylnaringenin |
prenylflavonoids |
|
Promotion of osteoblast differentiation and induction of osteoclast apoptosis [20] |
|
Epimedin B |
prenylflavonoids |
|
Inhibition of bone resorption, bone formation promotion and urinary calcium excretion blocking [21] |
|
Epimedin C |
prenylflavonoids |
|
Inhibition of bone resorption, bone formation promotion and urinary calcium excretion blocking [21] |
|
Tanshinones (dihydrotanshinone, tanshinone I, or tanshinone IIA) |
diterpenes |
|
Inhibition of the TRAP5b-expressing osteoclasts formation by suppressing RANKL-induced expression of c-fos and NFATc1 [22,23] |
|
Salvianolic acid A |
phenolic acids |
|
osteoblast differentiation modulation and osteoblast activity upregulation [24,25] |
|
Salvianolic acid B |
phenolic acids |
|
osteoblast differentiation modulation and osteoblast activity upregulation [24,25] |
|
Eudebeiolide B |
eudesmane-type sesquiterpenoid |
|
Osteoclastogenesis inhibition and ovariectomy-induced bone loss prevention by regulating RANKL-Induced NF-κB, c-Fos and Calcium Signaling [26] |
AKT—protein kinase B, BALP—bone-specific alkaline phosphatase, CTX—type I collagen cross-linked C-telopeptide, DPD—deoxypyridinoline, ER—oestrogen receptor, ERK—extracellular signal-regulated kinase, JNK—c-Jun N-terminal kinase, MAPK—mitogen-activated protein kinase, NFκB—nuclear factor-kappa B, NTX—type I collagen cross-linked N-telopeptide, PLC—phospholipase C, RANKL—Receptor Activator for Nuclear Factor κB Ligand, TRAP 5b—Tartrate-resistant acid phosphatase 5b.
|
Botanicals |
Population and Design |
Intervention |
Outcome |
Authors and References |
|---|---|---|---|---|
|
Soy isoflavones |
single open-group prospective clinical intervention; 42 postmenopausal women, |
three daily servings for 12 consecutive weeks of whole soy foods containing approximately 60 mg/day of isoflavones |
↓ NTX, ↑ osteocalcin |
Scheiber 2001 [27] |
|
Soy isoflavones |
RCT with 3 groups: soy rich diet, HRT, control; 187 healthy asymptomatic postmenopausal women aged 39–60, |
approximately 47 mg/day of isoflavones in diet group; duration: 6 moths |
↑ bone osteoblastic activity but not as effective as HRT in reducing the postmenopausal turnover, ↑ osteocalcin |
Chiechi 2002 [28] |
|
Soy isoflavones |
RCT with 3 groups: placebo, mid-dose, and high-dose, in pill form; 203 postmenopausal Chinese women aged 48 to 62, |
placebo (daily dose of 0 mg isoflavones + 500 mg calcium, n = 67) mid-dose (40 mg isoflavones + 500 mg calcium, n = 68) and high-dose (80 mg isoflavones + 500 mg calcium, n = 68); duration: 12months |
favourable effect on rates of change in BMC at the total hip and trochanter among later postmenopausal women (>4 y), in women with lower body weight (≤median, 55.5 kg), or among women with lower level of calcium intake (≤median, 1095 mg/day) |
Chen 2004 [29] |
|
Soy isoflavones |
RCT with 3 groups: placebo, mid-dose, and high-dose; 90 Chinese postmenopausal women aged 45–60 |
placebo (daily dose of 0 mg isoflavones) mid-dose (84 mg) and high dose (126 mg), 30 subjects/group; duration: 6months |
Retardation of lumbar and femoral bone loss at the lumbar spine (L1–L4) and bone resorption |
Ye 2006 [30] |
|
Soy isoflavones |
double-blind RCT with 2 groups: placebo, isoflavone conjugates in capsule form, 68 postmenopausal Japanese women |
Isoflavone group (75 mg of isoflavone conjugates/day), 34 subjects/group; duration: 12 months |
↑ serum equol in the equol producers but not in the nonproducers, preventive effects of isoflavones on hip BMD |
Wu 2007 [31] |
|
Soy isoflavones |
double-blind RCT with 3 groups: placebo, mid-dose, and high-dose in tablet form; 255 postmenopausal women aged 46–63 |
placebo (daily dose of 0 mg isoflavones) mid-dose (80 mg) and high dose (120 mg); duration: 3 years |
mild beneficial femoral BMD—and SSI |
Shedd-Wise 2011 [32] |
|
Soy isoflavones |
double-blind RCT with 2 groups: placebo, isoflavones in tablet form; 87 Korean postmenopausal women aged 45–60 |
Isoflavone group = 70 mg in 2 tablet per day (8.0 mg glycitin, 20 mg daidzein, and 12.4 mg genistin); duration: 12 weeks |
↑ serum BALP and osteocalcin |
Lee 2017 [33] |
|
Soy isoflavones |
RCT with 3 groups; placebo, HRT, phytoestrogens; 325 postmenopausal women |
HRT group (1 mg oestradiol and 0.5 mg norethisterone acetate p.o. daily, phytoestrogens group (40% standardized extract with 20 mg soy isoflavones (genistein and daidzein), two capsules = 40 mg p.o. daily; duration: 12 months |
no significant differences between the effectiveness of the HRT and phytoestrogen in terms of effects on BMD and bone resorption |
Tit 2018 [34] |
|
Soy isoflavones |
double-blind RCT with 3 groups: placebo, soy protein, soy protein + isoflavone in snack bar; 200 women within 2 years of the onset of their menopause |
placebo (isoflavone of less than 300 parts per billion) PI (15 g soy protein with 66 mg of isoflavones), SP (15 g soy protein alone, isoflavone free) daily, 100 women/group; duration: 6 months |
↓CTX with SPI supplementation compared to SP, ↓ P1NP with SPI supplementation |
Sathyapalan 2017 [35] |
|
Soy isoflavones |
double-blind RCT with 2 groups: placebo, isoflavones in form of tablet |
placebo (0 mg of isoflavones), isoflavones extracted from soy protein (200 mg daily = 4tablets) 248 multi-ethnic menopausal women aged 45 to 60; duration: 2 years |
not superior to placebo in preventing bone loss or in reducing bone turnover or menopausal symptoms in women in the first 5 years of menopause |
Levis 2011 [36] |
|
Soy isoflavones |
double-blind RCT with 2 groups: placebo, phytoestrogens; 202 postmenopausal women aged 60–75 |
placebo (milk protein), phytoestrogens (25.6 g soy protein containing 52 mg genistein, 41 mg daidzein and 6 mg glycetein (aglycone weights; duration: 12 months |
no significant differences for BALP, calcium, and phosphorus measurements. |
Kreijkamp-Kaspers 2004 [37] |
|
Soy isoflavones |
double-blind, multicentre RCT with 2 groups: isoflavone-enriched biscuits and bars and control biscuits and bars; 237 early postmenopausal women aged 53 ± 3y |
placebo group (biscuits and cereal bar), isoflavone- enriched foods (soy isoflavone concentrate containing 40–50% of isoflavones) providing a mean daily intake of 110 mg isoflavone aglycones/day; duration: 12 months |
isoflavone-enriched products did not alter lumbar and total body BMD or markers of bone formation and bone resorption |
Brink 2008 [38] |
|
Genistein |
double-blind RCT with 2 groups: placebo, genistein; 389 postmenopausal women |
placebo group (calcium and vitamin D, n = 191), genistein aglycone group (54 mg/day + calcium and vitamin D, n = 198) duration: 36 months |
↑lumbar and femoral BMD, ↓bone resorption markers (DPD, CTX, RANKL), ↑ bone formation markers (BALP, IGF-1 and OPG) |
|
|
Genistein |
double-blind RCT with 2 groups: placebo, genistein; 138 postmenopausal women (age 49–67 years) |
placebo (0mg of isoflavones, n = 67), genistein (54 mg/day, n = 71), duration: 24 months |
↑ femoral and lumbar BMD, improvement of the quantitative ultrasound parameters (stiffness index, amplitude-dependent speed of sound, and bone transmission time) |
Atteritano 2009 [41] |
|
Genistein |
double-blind RCT with 2 groups: placebo, geniVida™ bone blend group; 70 postmenopausal women |
placebo (calcium only, n = 28), genistein group = 30 mg/daygenistein + vitamin D3 (800 IU/days) + vitamin K1 (150 μg/days) + polyunsaturated fatty acids (1 g polyunsaturated fatty acids as ethyl ester: eicosapentaenoic acid/docosahexaenoic acid ratio = ~2/1, n = 30); duration: 6 months |
↑ BMD, ↑ BALP and NTX |
Lappe 2013 [42] |
|
Genistein |
double-blind RCT with 2 groups: placebo, genistein, 121 postmenopausal women |
placebo (1000 mg of calcium and 800 IU vitamin D3; n = 59), genistein aglycone group (54 mg/day + calcium, vitamin D3; n = 62, duration: 24 months |
↑ femoral and lumbar BMD, ↑ BALP |
Arcoraci 2017 [43] |
|
Red clover isoflavones (genistein, daidzein, formononetin, biochanin A) |
double-blind RCT with 4 groups: placebo, red clover isoflavone preparation (Rimostil) in 3 doses, 46 postmenopausal women |
placebo, Rimostil (phytoestrogens)—28.5 mg, 57 mg, or 85.5mg/day, duration: 6 months, |
↑ BMD after 57 mg and 85.5 mg/day |
Clifton-Bligh 2001 [44] |
|
Red clover isoflavones |
double-blind RCT with 2 groups: placebo, isoflavone supplement Promensil®; 205 pre-, peri-, and postmenopausal women aged 49–65 |
placebo, isoflavone supplement (providing 26 mg biochanin A, 16 mg formononetin, 1 mg genistein, 0.5 mg daidzein/daily); duration: 12 months |
↑ bone formation markers (BALP, P1NP), ↓ lumbar spine BMC and BMD |
Akinson 2004 [45] |
|
Red clover isoflavones |
double-blind, parallel RCT with 2 groups: placebo, red clover extract; 78 postmenopausal osteopenic women supplemented with calcium 1200 mg/day, magnesium 550 mg/day, calcitriol 25 µg/day |
placebo, red clover extract (60 mg isoflavone aglycones/day + probiotics); duration: 12 months |
↓ lumbar and femoral BMD loss, ↓ CTX |
Lambert 2017 [46] |
|
Red clover isoflavones |
double-blind RCT with 2 groups: placebo, red clover extract; 60 menopausal women |
placebo, red clover extract (daily dose of 150 mL containing 37.1 mg isoflavones = 33.8 mg as aglycones); duration: 12 weeks |
↑ spinal BMD |
Thorup 2015 [47] |
|
Red clover isoflavones |
double-blind RCT with 2 groups: placebo, standardized red clover isoflavone dietary supplement (Promensil®); 401 healthy women aged 35–70 years |
Placebo, red clover isoflavones (40 mg/day); duration: 36 months |
safe and well tolerated but no effect on BMD |
Powles 2008 [48] |
|
Red clover isoflavones |
double-blind RCT with 3 groups: placebo and 2 dietary supplements derived from red clover, 252 menopausal women ages 45–60 years |
placebo, Promensil® (82 mg total isoflavones), Rimostil® (57.2 mg total isoflavones), duration: 12 weeks |
no effect on bone turnover markers. |
Knudson Schult 2004 [49] |
|
Kudzu root (Pueraria candollei var. mirifica) |
double-blind RCT with 4 groups: placebo, 3 dose of Pueraria; 71 postmenopausal women aged 45 to 60 years |
placebo (n = 20), Pueraria mirifica in capsules (20, 30, or 50 mg once daily, n = 51); duration: 24 weeks |
↓ BALP |
Manonai 2008 [50] |
|
Kudzu root (Pueraria candollei var. mirifica) |
double-blind RCT with 2 groups 19 postmenopausal women |
placebo tablet, tablet containing 25 mg dried PM root powder, 4 tablets/day; duration: 2 months |
↓ ALP |
Okamura 2008 [51] |
|
Epimedium |
double-blind RCT with 2 groups: placebo, Epimedium-derived phytoestrogen flavonoids (EPF), 100 healthy late postmenopausal women |
placebo (n = 50), EPF group (n = 50; a daily dose of 60 mg Icariin, 15 mg daidzein, and 3 mg genistein), +300 mg calcium daily for both group; duration: 24 months |
↑ lumbar and femoral BMD, ↓ DPD, |
Zang 2007 [52] |
|
Dried plums |
RCT with 2 groups: placebo (dried apples), dried plums; 58 postmenopausal women |
placebo (dried apples 75 g daily), dried plums (100 g daily); duration: 3 months |
↑IGF-1, ↑ ALP, ↑ BALP |
Ajamandi 2002 [53] |
|
Dried plums |
RCT with 2 groups: placebo, dried plums, 160 postmenopausal women with osteopenia |
placebo (dried apples 75 g daily), dried plums (100 g daily) + 500 mg Calcium, 400 IU (10 μg) vitamin D daily for both group; duration: 12 months |
↑ ulnar and lumbar BMD, ↓ BALP |
Hooshmand 2011 [54] |
|
Dried plums |
RCT with 3 groups: placebo, 2 dose of dried plums, 48 older postmenopausal women |
control (0 g/day dried plum), dried plum (50 or 100 g/day dried plum), duration: 6 months |
↑ BMD, ↓ TRAP-5b, ↑ BALP/TRAP-5b ratio |
Hooshmand 2016 [55] |
|
Dried plums |
RCT with 3 groups: placebo, 2 dose of dried plums; 35 men between the ages of 55 and 80 with moderate bone loss |
control group (0g prunes), 100 g prunes daily, 50 g prunes daily, + multivitamin containing 450 mg calcium and 800 IU vitamin D for all group, duration: 3 months |
↓ osteocalcin, ↑ OPG/RANKL ratio |
Ajmandi 2020 [56] |
|
Horsetail (Equisetum arvense) |
Double blind RCT with 4 groups: control, placebo + horsetail extract, horsetail extract, calcium, 122 women in menopause for at least two years |
no treatment/control group (n = 29), placebo for 40 days and titrated horsetail extract for a further 40 days (n = 31), titrated dry horsetail extract for 80 days (n = 30); Calcium (Osteosil®) for 80 days (n = 32), After the 80-day initial study period, patients treated with titrated horsetail extract and with calcium continued treatment for one year |
↑ in the average densitometric values for the vertebra |
Corletto 1999 [57] |
|
Black cohosh (Cimcifuga racemosa) |
double-blind RCT with 3 groups: placebo, black cohosh, oestrogens; 62 postmenopausal women |
placebo, black cohosh (40 mg of herbal drug/day), conjugated oestrogens (0.6 mg/day); duration: 12 weeks. |
↑ osteoblast activity, weak estrogen-like activity, no significant effects on coagulation markers and liver enzymes |
Wuttke 2006 [58] |
|
Black cohosh (Cimcifuga racemosa) |
prospective clinical trial with 2 groups: untreated control, isopropanolic extract of Cimicifuga racemosa, 82 postmenopausal women |
control group (n = 37), isopropanolic extract of Cimicifuga racemosa (Remifemin®, 40 mg/day, n = 45), duration: 3 months |
↓NTX (marker of bone resorption), ↑ ALP (marker of bone formation) |
Garcia-Pérez 2009 [59] |
|
Black cohosh (Cimcifuga racemosa) |
RCT with 3 groups: control (CG), exercise group (EG), exercise and Cimicifuga racemosa (CR) supplementation group (EGCR), 128 early postmenopausal women |
CG (wellness control, n = 42), EG (n = 43), EGCR (40 mg/day of CR BNO 1055; n = 43), Calcium (1500 mg/d) + vitamin D (500 IE/d) supplementation for all participant duration:12 months |
CR (CR BNO 1055) did not enhance positive effects of exercise on BMD at the lumbar spine |
Bebenek 2010 [60] |
|
Labisia pumila and Eurycoma longifolia |
double-blind RCT with 2 groups: placebo, Nu-femme™, 119 healthy women aged 41–55 years experiencing peri-menopausal or menopausal symptoms |
placebo (n = 59), herbal formulation (Nu-femme™, n = 60) = 200mg Labisia pumila (SLP+®) + 50mg Eurycoma longifolia (Physta®); duration: 24 weeks |
No significant effect on bone formation (BALP) and resorption (NTX) markers |
Chinnappan 2020 [61] |
ALP—alkaline phosphatase, BALP—bone-specific alkaline phosphatase, BMC—bone mineral content, BMD—bone mineral density, CTX—type I collagen crosslinked beta C-telopeptide, DPD—deoxypyridinoline, HRT—hormonal replacement therapy, IGF-1– insulin-like growth factor 1, NTX—type I collagen crosslinked N- telopeptide, OPG—osteoprotegerin, P1NP—type I procollagen-N-propeptide, RANKL—Receptor Activator for Nuclear Factor κB Ligand, SSI—strength strain index, ↑—increased, ↓—decreased
2. Phytoestrogens
2.1. Isoflavones
|
Aglycones |
Glycosides |
Acetylglycosides |
Malonyl Isoflavone Glycosides |
|---|---|---|---|
|
Daidzein |
Daidzin |
Acetyldaidzin |
Malonyldaidzin |
|
Genistein |
Genistin |
Acetylgenistin |
Malonylgenistin |
|
Glycitein |
Glycitin |
Acetylglycitin |
Malonylglycitin |
|
Biochanin A |
Sissostrin |
Malonylsissostrin |
|
|
Formononetin |
Ononin |
Malonylononin |
|
|
Daidzein |
Daidzin |
Acetyldaidzin |
Malonyldaidzin |
2.2. Other Plants Containing Phytoestrogens Investigated in Osteoporosis Treatment
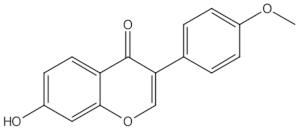
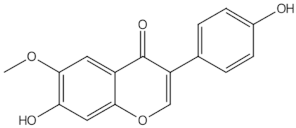
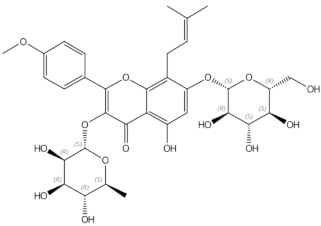
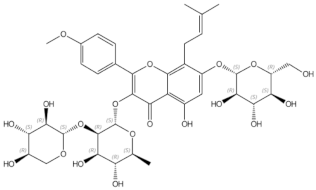
2.2.1. Epimedium (Berberidaceae)
Epimedium in Clinical Trials
Epimedium in Animal Models and In Vitro Studies
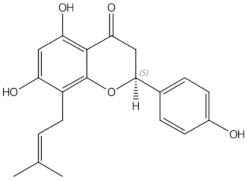
2.2.2. Hop (Humulus lupulus L.)
This entry is adapted from the peer-reviewed paper 10.3390/nu13051609