Despite the clinical implementation of novel treatments like kinase inhibitors, hypomethylating agents or pathway modulators during the last decades, several hematological malignancies still face a poor prognosis. Relapsed or refractory courses are particularly difficult to manage, often exhibiting multiple drug resistances and intimidating survival rates [1–4]. The FDA approval of the cyclin dependent kinase (CDK) 4/6 inhibitors (CDKIs) palbociclib, ribociclib, and abemaciclib for hormone receptor positive and human epidermal growth factor receptor 2 negative locally advanced or metastatic breast cancer treatment led to numerous promising clinical studies investigating CDKIs in solid neoplasms. This provides a rationale for the emerging intensive testing in hematological malignancies, raising hopes for improving patients’ prognoses. Given their wide spectrum of cellular modulation including cell cycle control, transcription, DNA repair, epigenetic regulation, proliferation, and apoptosis, CDKs represent promising molecular targets for leukemia and lymphoma treatment [5].
Cell cycle regulation is a key mechanism to prevent malignant cell proliferation and uncontrolled cell division. Aleem and Arceci reviewed the roles of CDKs in controlling cell cycle and development of hematological malignancies in detail [6]. Here, we focus on present and future therapeutic approaches to overcome CDK-influenced cell cycle control malfunction (Table S1) and will thus only offer a short introduction on CDK pathways and their role on leukemogenesis. In brief, cell cycle control is mainly mediated by serine/threonine CDKs acting as catalytic subunit when activated by their respective cyclins. CDK activity is further modulated by physiological CDKIs and posttranslational modification, resulting in transcriptional regulation, DNA damage repair mechanisms, metabolism, or epigenetic processes. In addition to the “classical” CDKs directly influencing the cell cycle, further kinases act as indirect modulators to regulate transcription or epigenetic signaling [6].
Hematopoietic stem cells are relatively quiescent to prevent stem cell exhaustion [7–9]. Once these stem cells initiate cell cycle induction, the cells proliferate extensively to provide hematopoiesis [10]. Therefore, the cell cycle of hematopoietic stem cells must be controlled thoroughly. Dysregulation of CDKs and associated cyclins is frequently observed in hematological malignancies. CDK6 is predominantly expressed in hematopoietic cell types and loss of CDK6 results in impaired generation of several blood cell types. In contrast, overexpression and chromosomal translocation of CDK6 is observed in acute lymphoblastic leukemia (ALL) and lymphoma. CDK4/6 inhibition is achieved by physiological CDKI p16INK4A, the most frequently deleted locus in human cancer. Translocations of the MLL gene locus are common in acute myeloid leukemia (AML) and account for most infant ALL cases. CDK6 is a direct target of MLL fusion proteins and thus activated. Finally, CDK6 can be activated by FLT3-ITD-mediated down-regulation of cyclins D2 and D3. Besides CDK6, other CDKs are also involved in leukemogenesis. For example, the AML driver mutation FLT3-ITD is an activator of CDK1. Further, mutations and deletions of the cyclin C and CDK19 locus on 6q21 result in altered Notch1 regulation especially in T-ALL [6].
While the pan CDKI flavopiridol was the first CDK inhibitor applied clinically [
11], recent CDKIs are more specific, with most drugs targeting only a subset of CDKs. CDK6 has a central role in hematopoiesis and CDK6-deficient mice show reduced production of erythrocytes, granulocytes, macrophages, neutrophils, and thrombocytes, as well as thymic atrophy [
6]. Neutropenia is therefore the most common and dose-limiting adverse event in the clinical use of CDK6 inhibitors [
12]. CDK6 is also a direct target of MLL fusion proteins which are common in AML and ALL [
13,
14]. This results in transcriptional activation of CDK6 and subsequent initiation of leukemic processes [
14,
15,
16]. Further, CDK6 can be activated via
FLT3-ITD-mediated upregulation of cyclins D2 and D3 [
17]. In mantle cell lymphoma, the characteristic t(11;14) translocation induces ectopic cyclin D1 expression, also resulting in CDK6 and CDK4 upregulation [
18]. In pediatric B-ALL, p16
INK4A deletions, especially occurring during relapse, are a key feature of dysfunctional CDK4/6 control and an associated dismal prognosis [
19].
1.1. Palbociclib
The CDK4/6 inhibitor palbociclib was widely evaluated in solid tumors and is now also analyzed in a variety of hematopoietic malignancies. It demonstrated significant pre-clinical in vitro and in vivo efficacy in AML cells with
FLT3-ITD [
17,
20] and TKD mutations [
21],
RUNX1/ETO translocation [
22] and
MLL rearrangement [
15,
23]. In a recent study investigating de novo transformation of granulocyte/macrophage progenitor cells to AML, Chen et al. demonstrated that transient palbociclib application is capable of halting progenitor cell proliferation and preferentially abrogated the most proliferative progenitor cell subsets. Palbociclib further reduced the progenitor cell transformation in vivo, resulting in reduced AML burden and prolonged survival. This suggests that cell cycle inhibition decreases the likelihood of malignant transformation in vivo [
24].
In T-ALL cells as well as B-ALL cells featuring
MLL or BCR-ABL1 rearrangements, palbociclib controlled cell growth via G1 arrest and Rb dephosphorylation both in vitro and in vivo [
14,
25,
26,
27,
28]. A phase I clinical trial investigating palbociclib in relapsed ALL children is currently underway.
Similar promising antitumoral effects were observed in multiple myeloma, anaplastic large-cell lymphoma, and mantle cell lymphoma, where palbociclib was also capable of overcoming ibrutinib resistance [
29,
30,
31,
32,
33,
34]. In a study with 17 relapsed mantle cell lymphoma patients, palbociclib achieved one complete remission (CR) and two partial responses (PR), five patients had a progression-free survival of at least one year with reduced tumor metabolism and proliferation [
35]. A phase I study in non-Hodgkin lymphoma showed stable disease (SD) in one third of the participants and two out of 68 patients had PR [
36].
Palbociclib was then evaluated in several combinations both preclinically and clinically. The combination with proteasome inhibitor bortezomib was effective in an immunocompetent myeloma mouse model. Inhibition of CDK4/6 by palbociclib induced G1 arrest and enhanced bortezomib susceptibility via increased mitochondrial depolarization [
30]. This combination was also evaluated in a clinical phase I/II trial in refractory/relapsed (R/R) myeloma together with dexamethasone, demonstrating an overall response rate of 20% and SD in 44% of the participants [
37]. A phase I trial in R/R mantle cell lymphoma with palbociclib and bortezomib achieved CR in one out of 19 patients [
38]. Combined palbociclib and ibrutinib treatment has been evaluated in R/R mantle cell lymphoma. From 27 patients in this phase I study, 67% responded and 37% had CR [
39].
Further combination partners are evaluated preclinically in vitro and in vivo. In AML, the cytarabine dose could be reduced after palbociclib priming () [
40]. Besides cell cycle regulation, CDK6 also controls gene expression of oncogenic kinases by directly binding promoter sites. These include, among others, AURORA and AKT, both of which are known mediators of drug resistance.
FLT3 mutations can further contribute to pathway activation in AML. Combined palbociclib and pan-AURORA kinase inhibitor danusertib or AKT inhibitor MK-2206 treatment resulted in synergistic anti-leukemic effects in
FLT3-ITD and TKD mutated AML cells [
21].
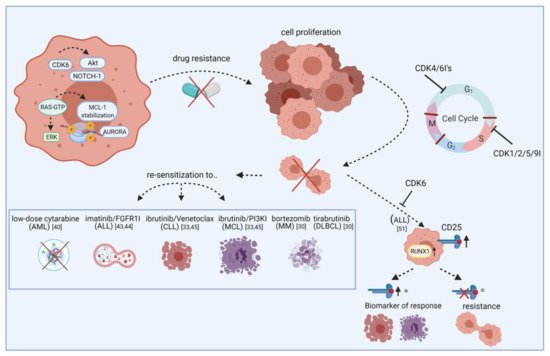
Figure 1. Re-sensitization of drug-resistant cells by CDK inhibitors. The scheme illustrates how CDKIs can conquer resistance of leukemic blasts towards targeted agents. Leukemic cells show frequent overexpression or constitutive activation of oncogenic kinases like AURORA and AKT. While these kinases mediate drug resistance via apoptosis inhibition or increased tumor cell proliferation, blocking specific CDKIs may help to make cells more vulnerable to certain approved drugs, including cytarabine, imatinib, and ibrutinib. This effect is likely mediated by cell cycle checkpoint blockade, resulting in impaired cell division and proliferation. Additionally, blocking CDKs induces CD25 abundance on ALL cells and this may act as a biomarker for response. Vice versa, lack of CD25 expression indicates resistance. FGFR1I, fibroblast growth factor receptor 1 inhibitor; PI3KI, phosphatidyl inositol 3 kinase inhibitor; MCL, mantle cell lymphoma; MM, multiple myeloma; DLBCL, diffuse large B-cell lymphoma. Created with BioRender.
Although there is a clear connection between BCR-ABL1 fusion and cell cycle regulation, only very limited data is available for the evaluation of CDKIs in chronic myeloid leukemia (CML). Rangatia and Bonnet have shown that lack of BCR-ABL1 leads to G1 phase arrest and a decrease in cyclin D1. Physiological CDKIs p21 and p27 subsequently exhibited increased gene expression [
41]. Schneeweiss-Gleixner et al. recently reported that palbociclib synergizes with tyrosine kinase inhibitor ponatinib in BCR-ABL1
T315I mutated CML, via reducing proliferation and inducing G1 arrest. This is of importance because tyrosine kinase inhibitor resistance is frequently observed in this CML subtype, leading to challenging clinical problems [
42]. In ALL, palbociclib synergizes with fibroblast growth factor receptor 1 inhibitor PD-173074 and imatinib (Gleevec) [
43,
44]. Increased apoptosis rates compared to palbociclib mono application were achieved with PI3Kδ inhibitor GS-1101 and BET protein bromodomain antagonist JQ1 in mantle cell lymphoma [
45,
46]. In myeloma and diffuse large B-cell lymphoma, synergism was achieved with dexamethasone and Bruton’s tyrosine kinase inhibitor tirabrutinib [
29,
47].
To demonstrate their anti-leukemic potential, CDK4/6 inhibitors rely on expression of downstream signaling protein Rb. Intrinsic or acquired lack of Rb function results in resistance towards therapeutic CDK4/6 targeting [
12]. Additionally, palbociclib treatment can lead to p27 downregulation, thus reactivating CDK2 and cell cycle progression. Transcription factor
FOXO3A can additionally control
p27 gene expression. However, it was not influenced in palbociclib-resistant cells; ruling out the possibility of
FOXO3A directly induced
p27 downregulation. Palbociclib-sensitive cell lines exhibited a similar drop in
p27 gene expression, while protein abundance remained stable. Hence, the
p27-downregulating effect is rather due to posttranslational modification than reduced
p27 gene expression () [
17].
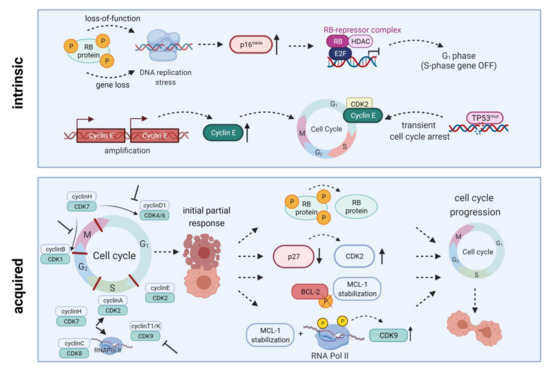
Figure 2. Intrinsic and acquired resistance mechanisms towards CDKIs. Intrinsic resistance is driven by lack of functional Rb protein, leading to DNA replication stress, high-level expression of endogenous CDK4/6 inhibitor p16INK4A, and inactivation of E2F-regulated genes. Another resistance mechanism is based on Cyclin E amplification and overexpression driving cell cycle progression. Quite in line, TP53 mutations are generally associated with low response to CDK inhibition. Acquired resistance mechanisms include Rb loss, CDK2 reactivation upon p27 downregulation, a truncated BCL-2 protein lacking the phosphorylation loop domain, as well as prolonged MCL-1 stability along with RNA polymerase II phosphorylation and CDK9 kinase domain up-regulation. All these mechanisms may ultimately contribute to cell cycle progression and thus CDKI resistance. HDAC, histone deacetylase; E2F, E2F transcription factor family; RNA Pol II, RNA polymerase II. Created with BioRender.
1.2. Ribociclib
Ribociclib (LEE011) is another CDK4/6 inhibitor that demonstrated significant anti-proliferative and apoptosis-inducing effects in AML and B-ALL cell lines and primary samples, probably mediated via G1 arrest and senescence [
48]. A recent study on pediatric B-ALL evaluated ribociclib in combination with dexamethasone. They found significant basal overexpression of CDK6 in B-ALL cells. Subsequent inhibition of CDK4/6 using ribociclib resulted in G1 phase arrest, reduced cell proliferation, and apoptosis induction accompanied by Rb dephosphorylation. Adding dexamethasone synergistically improved anti-neoplastic effects in B-ALL cell lines as well as primary samples [
49].
Pikman et al. investigated ribociclib in
NOTCH1-mutant and wildtype T-ALL and found both subtypes sensitive to the inhibitor. The combination with glucocorticoid dexamethasone and mTOR inhibitor everolimus acted synergistically in vitro and in vivo. In contrast, antagonistic effects were observed with several drugs used in T-ALL standard chemotherapy including methotrexate, mercaptopurine, asparaginase, or doxorubicin [
50].
In T-ALL, CDK6 is required for AKT- or Notch1-induced leukemia initiation. Jena et al. demonstrated increased
CD25 and
RUNX1 expression upon CDK6 inhibition and that CD25 ablation results in T-ALL leukemogenesis. They further showed that CD25 mediates the therapeutic response to ribociclib, suggesting CD25 deletion as a potential mechanism of resistance to CDK6 inhibitors as well as predictive marker for ribociclib response () [
51]. A clinical phase I study examined ribociclib in solid neoplasms and lymphoma and found SD in ten out of 70 patients [
52].
1.3. Abemaciclib
While ribociclib was mainly analyzed in acute leukemias, abemaciclib (LY2835219) has been more widely evaluated in lymphoma. Here, inhibited proliferation and induced apoptosis was reported in germinal center-derived B-cell lymphoma cell lines, but not in activated B-cell like diffuse large B-cell lymphoma cell lines [
53]. In a clinical trial, 71% of the participating R/R mantle cell lymphoma patients achieved SD and 36% had PR [
54]. A subsequent phase II study with 28 patients reached CR in two patients and an overall response rate of 36% with an overall survival of 16 months [
55]. Another clinical trial is currently evaluating abemaciclib with fulvestrant in advanced or metastatic solid tumors and lymphomas.
Further preclinical studies found abemaciclib effective in
MLL-rearranged AML cell lines and xenografts [
56] as well as myeloma cell lines [
57]. Nakatani et al. recently identified that t(8;21) rearranged AML cell lines are more vulnerable to palbociclib and abemaciclib than non-t(8;21) AML cells [
58]. Molecularly, this is due to higher cyclin D2 levels, a common response marker towards abemaciclib. In t(8;21) rearranged AML cells, abemaciclib induced the expected G1 arrest, leading to impaired cell proliferation and decreased MAPK and AKT pathway signaling. Another interesting finding of this study was autophagosome formation which significantly increased apoptosis induction upon combined application of abemaciclib and autophagy inhibitors [
58].