Those properties attracted the interest of researchers to polyphenols, and many studies highlighted their potential role in the prevention and treatment of various pathological conditions connected to oxidative stress and inflammation, like cancer and cardiovascular and neurodegenerative disorders, and also of pollutant-induced cell damage [
8,
9,
10,
11,
12]. Moreover, those food products are relatively abundant in the human diet, and several foods and beverages can provide more than 1 mg of polyphenolic content per serving, as shown by the study of Pérez–Jiménez et al. (2010) based on data from the Phenol-Explorer database [
13]. As an example, by using the same database, Godos et al. (2017) estimated that an Italian study population had a mean intake of approximately 660 mg of polyphenols per day, obtained from nuts, tea, coffee, cherries, citrus fruits, vegetables, chocolate, and red wine [
14], all regular constituents of the Mediterranean diet [
15], included in the list of the Intangible Cultural Heritage of Humanity by UNESCO. In fact, olive-oil components were described as relevant pharmacological molecules [
16]. However, major dietary sources of polyphenols may vary depending on the traditional diets adopted in various countries, thus, in Northern and Eastern European countries, the main dietary sources of polyphenols are represented mostly by beverages, such as coffee and tea [
17,
18], while in Southern European and Mediterranean countries, important dietary sources may be nuts, olive oil, fruits, and vegetables [
14,
19].
Novel data expanded on the initial antioxidant-based mechanism of polyphenols’ action by showing that they are also able to modulate several cell-signaling pathways and mediators in a wide range of human pathologies. In a recent publication, Patel et al. (2019) revised the pharmacological applications of curcumin in several diseases [
22], as well as a wide range of pleiotropic actions in the modulation of cell-signal molecules. In diabetes, tea polyphenols were able to reduce the senescence of glomerular mesangial cells by regulating the activity of miR-126/Akt-p53-p21 pathways [
23]. The consumption of flavonoid compounds seemed to also have a beneficial effect on colon-cancer prevention by modulating lysosome enzymes, increasing the expression of apoptotic factors like Bax, Bcl2, and caspase-3 in cancer cells, and regulating cellular respiratory and mitochondrial enzymes [
11]. A recent review also pointed out the beneficial role of dietary polyphenols quercetin and epigallocatechin gallate (EGCG) in the prevention and treatment of obesity, with important impact on the prevention of cardiovascular diseases. Quercetin appeared to modulate adipogenic pathways like the adenosine-5′-monophosphate (AMP)-activated protein kinase, and upregulate the levels of phosphorylated AMP-activated protein kinase (AMPK) and its substrate, acetyl-CoA carboxylase, in 3T3-L1 preadipocytes, while EGCG appeared to inhibit the proliferation and differentiation of 3T3-L1 preadipocytes in mature adipocytes by arresting the cell cycle [
24]. However, it is still controversial whether or not these products can naturally increase intrinsic brain defenses and avoid, or at least reduce, the initial insults that lead to the neurodegenerative process.
2. Beneficial Effects of Polyphenols and Neuroprotection
Neurodegenerative diseases are typically characterized as pathological conditions where particular groups of neurons are damaged or lost, disturbing the normal function of the central nervous system, either by impairing cognitive functions, motor functions, or both. Many of those illnesses are commonly associated with aging, but it is currently known that neurodegeneration develops in a subclinical form over years, with neuronal death occurring progressively over a lifetime, much before the first clinical signs are noticeable. Current predictors indicate a continuous increase in dementia cases that, between 2005 and 2030, may reach about 50% of the aged population [
25]. Numerous studies [
26,
27,
28,
29] have been dedicated to the cellular mechanisms for neurodegeneration in several pathological conditions, such as Alzheimer’s, Parkinson’s, and Huntington’s disease, as well as amyotrophic lateral sclerosis. However, there are currently no effective therapies available to treat such diseases besides symptom amelioration [
30,
31].
In spite of their specific pathways, many of those conditions share common mechanisms, such as neuroinflammation [
32] and oxidative stress [
29,
33]. In fact, the possible role of reduced expression or imbalance of oxidative-stress regulatory genes in aging and neurodegeneration, as well as the possible protection by antioxidants, was already reviewed [
34]. Therefore, any strategies that can delay or prevent the onset of the disease, conveying neuroprotection, may be as important as the ones designed to treat it. The notion that diet can have a crucial role as one of those strategies has recently been proposed, leading to several studies focused on the importance of nutritional consumption of natural products, as food itself or as food supplements, that may convey neuroprotection [
35,
36].
One of the first indications of biological activity from food-derived molecules was the discovery of the antibacterial properties of curcumin, published in
Nature in the late 1940s by Schraufstatter and Bernt [
37]. Other food polyphenols, particularly resveratrol, also attracted the attention of researchers, as suggested by the possible association between red-wine consumption in France and the low incidences of coronary heart disease [
38]. This association could be explained by the antioxidative properties of food polyphenols, in this case resveratrol, that were also found to convey neuroprotective activity [
38,
39,
40].
Several studies using polyphenols, particularly the ones from red wine or green tea [
41], have focused on their neuroprotective role in most neurodegenerative diseases, like recently described neuroprotection by epigallocatechin gallate (EGCG) from amyloid-beta-mediated neurotoxicity [
42]. These studies also showed the ability of EGCG to inhibit Bax and cytochrome c translocation and autophagic pathways by increasing LC3-II [
43], and to modulate mitochondrial functions [
44]. It was also demonstrated that EGCG is able to significantly cross the human blood–brain barrier (BBB) model and protect cortical cultured neurons from oxidative-stress-induced cell death [
45]. In fact, recent studies suggested that some flavonoids are indeed able to reach the brain [
46], and it is now important to clarify by which mechanisms they exert neuroprotection.
Further examples of polyphenols pinpointed as promising molecules in the prevention and treatment of neurodegenerative diseases can be found in the literature [
21,
47,
48,
49,
50,
51]. One of the most relevant examples is resveratrol, shown to have neuroprotective properties by decreasing microglia-induced neuroinflammation, protecting the brain against hypoxic–ischemic damage and ameliorating cognitive function in the Alzheimer’s disease model [
38,
39,
40,
52]. It also seems to decrease age-related cognitive decline and increase cognitive function through SIRT1 modulation that, among other important functions, seems to modulate the growth of dendrites and axons [
53]. The neuroprotective effects of resveratrol go beyond the central nervous system, since it also seemed to reduce NFκB-mediated neuroinflammation and endoplasmic reticulum stress in an ischemia–reperfusion model of vasculitis peripheral neuropathy; this condition arises from an obstruction in the blood vessels supplying peripheral nerves due to inflammation and may be related to neuropathic pain [
54]. Protein kinase C gamma was also described as another target for resveratrol and EGCG in a way that its activation is associated with neuroprotection [
55]. It is, however, interesting to verify that, as expected, the beneficial roles of polyphenols are not all equal in intensity and vary among different food sources. For example, it was found in an Alzheimer’s rat model that better neuroprotection was achieved by supplementation with green tea than with black tea or red wine [
56]. In a similar mouse model, pomegranate juice seemed to decrease amyloid deposition and improve behavior tests after food supplementation [
10]. Interestingly, it was recently published that blueberry supplementation of rat food was able to reduce microglial inflammatory reaction due to the graft transplant [
57], particularly in aged rats, and also to protect neural cells from oxidative stress and attenuate microglia activation [
58]. A similar anti-inflammatory effect was observed in vitro after the lipopolysaccharide (LPS) stimulation of BV-2 cells [
59], a microglia cell line. Exciting results were also achieved with the well-known curcumin in Parkinson’s disease [
60], as well as for medicinal plants used in traditional medicine, like
Centella asiatica, which were shown to reduce mitochondrial dysfunction and oxidative stress while improving cognitive function in an Alzheimer’s in vivo model [
51].
Furthermore, a growing field of research illustrates the possibility for epigenetic modulation by dietary consumption of polyphenols, namely, on the modulation of pro- and anti-inflammatory microRNAs [
61]. An example of those properties is neuroprotection via autophagy modulation in a prion disease model [
43]. A recent review [
62] highlighted the epigenetic modulation of curcumin, including the inhibition of DNA methyltransferases, regulation of histone modifications through regulation of histone acetyltransferases and histone deacetylases (HDACs), as well as regulation of microRNAs. The modulation of endothelial-cell inflammation through the epigenetic regulation of NF-κB target genes by EGCG, proposed by Liu et al. [
9] as a beneficial agent against environmental pollutants’ vascular toxicity, may also have an important impact on the protection of BBB function in neurodegenerative diseases.
Interestingly, a relationship between proliferation in neurogenic niches and nutrition may also exist [
63], as well as a relationship between neurogenesis impairment and neuroinflammation [
64]. These findings raise the possibility that modulation of neuronal precursors’ niches may minimize the decline that could be associated with age, or the neurodegenerative disease itself, and constitute a promising field for further investigation.
In sum, the inclusion of phenolic compounds in the diet or their use as supplements, nutraceuticals, or pharmacological drugs, seems to be promising in the prevention of several different pathologies, namely, neurodegenerative diseases. An extensive list of such diseases (including depression, glutamate excitotoxicity, epilepsy, hearing, and vision disturbances, and neurodegenerative diseases), as well as in vitro, ex vivo, and in vivo studies that evaluated the action mechanisms of phenolic acids in those conditions, were reviewed by Szwajgier et al. [
65]. Interesting studies were conducted on the general population exploring the association between dietary polyphenols and depressive symptoms leading to similar results, including the potential role of phenolic acids [
66,
67]. However, the overconsumption of polyphenols may raise safety concerns due to accumulation of high levels of these molecules in the organism, particularly if we consider the loose regulatory legislation regarding the commercialization and use of food supplements.
The proposed benefits of polyphenols, either as protective/prophylactic substances or as therapeutic molecules, may be achieved by the consumption of a natural polyphenol-enriched diet, and by their use as food supplements or formulation as pharmaceutical drugs/nutraceuticals [
68]. It was also proved that the health effects of polyphenols depend on the consumed amount and on their bioavailability [
69].
3. Conclusions
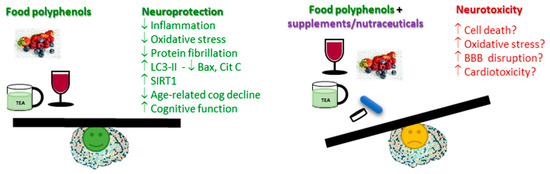
Polyphenols are promising molecules for the prevention and possibly the treatment of many human pathologies, namely, neurodegenerative diseases. However, like any pharmaceutical drug, they might show parallel adverse effects and/or toxicity, particularly due to the accumulation of high levels in the organism (). More studies are needed to discern the relationship between consumption and safe plasma concentrations that are beneficial. Until further studies are performed, a more natural consumption of polyphenol-rich products, like fruits, vegetables, tea, and coffee, is the most beneficial, while the overconsumption of food supplements advertised as polyphenols or polyphenol-rich, but mostly still poorly controlled by regulatory agencies, may lead to higher circulating levels and higher risk for adverse effects. Nevertheless, such supplements may be a useful resource when dietary food sources are not available.
Figure 2. Summary of key mechanisms and actions from polyphenol neuroprotection, also highlighting possible safety concerns derived from polyphenol overconsumption.
Modifying nutritional habits by the regular inclusion of polyphenol-rich fresh foods, like red fruits, tea, and natural juices, rather than with the excessive consumption of concentrated supplements, may have the most beneficial effect in long-term neuroprotection by increasing the organism’s adaptive natural defenses and modulating several pathological mechanisms involved in neurodegeneration.