Liver fibrosis is the consequence of different inflammatory processes occurring in any chronic liver disease. Its progression determines the development of cirrhosis and portal hypertension. The natural history of cirrhosis is characterized by a compensated phase, with or without portal hypertension, and a decompensated phase characterized by the appearance of major complications, such as ascites, portal hypertensive bleeding, encephalopathy, and jaundice. Malnutrition is frequent in patients with liver cirrhosis, which progresses in parallel with the worsening of the disease. Its etiology is multifactorial, given the great impact of liver disease on multiple processes related to nutrition.
- liver cirrhosis
- malnutrition
- malabsorption
- vitamins
- minerals
- sarcopenia
- liver transplant
- pancreatic exocrine insufficiency
- nutritional assessment
1. Consequences of Liver Disease on Nutritional Status
| Nutritional Consequence [Ref.] | Mechanisms in Chronic Liver Disease |
|---|---|
| 1. Impaired dietary intake [2][3] | Anorexia, dysgeusia, abdominal pain, bloating, early satiety secondary to ascites, prescription of restrictive diets, alcohol consumption |
| 2. Altered macro and micronutrient metabolism [4][5][6][7][8][9][10][11] | Lack of glycogen and vitamin storage, breakdown of fat and proteins as the principal energy source, decrease of vitamin and mineral levels |
| 3. Energy metabolism disturbances [12] | Hypermetabolic state, impaired glucose and lipid metabolism, sedentary lifestyle |
| 4. Increase in energy expenditure [13][14] | Increased catecholamines, malnutrition, immune compromise |
| 5. Nutrient malabsorption [15][16] | Decreased bile production, cholestasis, portosystemic shunting, portal hypertension gastropathy and enteropathy, small intestinal bacterial overgrowth, drug-related diarrhea |
| 6. Sarcopenia and muscle function [17][18][19] | Proteolysis as the energy source, inhibition of muscle growth, muscle autophagy, proinflammatory state |
| 7. Metabolic osteopathy [20] | Decrease in bone formation, increased bone resorption, dysbiosis, vitamin K and D deficiencies |
1.1. Impaired Dietary Intake
1.2. Altered Macro and Micronutrients Metabolism
1.2.1. Plasma Proteins
1.2.2. Vitamins and Minerals
| Vitamin [Ref.] | Liver Role | Deficiency and Liver Disease | |
|---|---|---|---|
| Fat-soluble vitamins | |||
| A (retinol) [5][6][7] | Production of RBP4 (transporter) Main storage in HSc (80%) |
Lost in vitamin A storage through the transformation of HSc into myofibroblasts. Deficiency is associated with nyctalopia (night blindness) and with hepatic encephalopathy | |
| D [5][6][7][9] | 25-hydroxylation site Production of binding proteins | Deficiency is associated with fibrosis, liver dysfunction, and mortality | |
| K [5][6][7] | Absorption of vitamin K trough bile acids | Deficiency is associated with coagulopathy and bone disease through an inadequate carboxylation of bone matrix proteins | |
| E [5][6][7] | Absorption of vitamin E trough bile acids | Deficiency is associated with hemolytic anemia, creatinuria, and neuronal degeneration | |
| Water-soluble vitamins | |||
| B [5][6][7] | B1 (thiamine) |
Normal thiamine function | Lost in activation and transport. Deficiency is associated with neurologic dysfunction (Wernicke encephalopathy) and high-output heart failure (wet beriberi) |
| B2 (riboflavin) |
Storage of riboflavin | Inadequate intake, increased utilization, and deficient storage. Deficiency is associated with inflammation of the gums and sores | |
| B6 (pyridoxine) |
Storage of pyridoxine | Deficiency is associated with anemia and neutropenia | |
| B9 (folate) |
Storage of folate | Deficiency is associated with anemia and macrocytosis | |
| B12 (cobalamin) |
Storage of cobalamin | Deficiency is associated with anemia and neutropenia | |
| C [5][6][7] | Storage of vitamin C | Deficiency is common in MAFLD. Deficiency is associated with bleeding, joint pain, and an increase of free radicals | |
| Minerals | |||
| Zinc (Zn) [5][6][7] | Absorption of Zn | Inadequate dietary intake, impaired absorption, and an increase in urinary loss. Deficiency is associated with hepatic encephalopathy and alterations in taste and smell | |
| Magnesium (Mg) [5][6][7] | Transport of Mg | Impaired transport and decrease intake. Deficiency is associated with dysgeusia, decreased appetite, muscle cramps, and weakness | |
| Manganese (Mn) [10] | Absorption trough bile acid production | Elevated if there is a decrease in biliary excretion Deficiency is associated with brain accumulation and parkinsonism |
|
| Carnitine [11] | Metabolism of carnitine | Poor intake. Deficiency is associated with muscle cramps | |
| Selenium (Se) [5][6][7] | Metabolism of Se | Deficiency related to severity liver disease Deficiency is associated with insulin resistance |
|
| Iron (Fe) [5][6][7] | Metabolism of Fe | Overload in alcoholic liver disease. Deficiency is associated with hepatic overload, fibrosis, and dysfunction | |
1.3. Energy Metabolism Disturbances
1.4. Increase in Energy Expenditure
1.5. Nutrient Malabsorption
1.6. Sarcopenia and Muscle Function
1.7. Metabolic Osteopathy
1.8. Interplay between MAFLD and Diet
2. Nutritional Assessment of Patients with Chronic Liver Disease
2.1. Nutritional Screening and Risk of Malnutrition
| Screening Tool [Ref.] | Target Population | Variables | Strengths and Weaknesses | Usefulness in Patients with Liver Cirrhosis |
|---|---|---|---|---|
| MST [27] | Hospitalized patients | 1—Weight loss 2—Food intake 3—Appetite |
Quick and easy No calculations No training Self-administered |
May be inaccurate due to fluid overload. Low sensitivity in patients with liver cirrhosis. |
| MUST [28] | Hospitalized patients and outpatients | 1—BMI 2—Weight loss 3—Acute illness and impact on dietary |
Quick and easy Adds acute illness Offers advice |
May be inaccurate due to fluid overload. Low sensitivity in patients with liver cirrhosis. |
| MNA-SF [29] | Elderly patients | 1—Weight loss 2—Appetite 3—Mobility 4—Neuropsycho problems 5—BMI 6—Acute illness |
Full evaluation, not only nutritional aspects BMI can be replaced by calf diameter |
Good performance in liver cirrhosis. High sensitivity and good specificity. |
| NRS-2002 [30] | Hospitalized patients | 1—BMI 2—Weight loss 3—Food intake 4—Illness severity |
Adds illness severity and age | May be inaccurate due to fluid overload. Low sensitivity in liver cirrhosis. High specificity |
| CONUT [31] | Informatic tool Hospitalized patients and outpatients |
1—Albumin 2—Cholesterol 3—Lymphocytes 4—Age 5—Illness severity 6—Length of illness 7—Treatment |
Automated screening of large populations Blood test required Low specificity |
Predictor of survival and complications after liver resection. Predictor of survival in end-stage liver disease. |
| SNAQ [32] | Hospitalized patients and outpatients | 1—Weight loss 2—Appetite 3—Nutritional supplements 4—BMI 5—Albumin 6—Lymphocytes |
Simple and quick Provides a recommendation Blood test required |
Limited data on the population with liver cirrhosis, but correlation with the Child–Pugh stage. |
| RFH-NPT [33] | Patients with liver cirrhosis | 1—Transplant 2—Fluid overload 3—Weight loss 4—Food intake 5—BMI (in absence of fluid overload) 6—Acute illness |
Adds transplantation Reduces the impact of fluid retention Adds acute illness |
Superior results compared to other tests in liver cirrhosis. High sensitivity and specificity. |
| LDUST [32] | Patients with liver cirrhosis | 1—Food intake, 2—Weight loss 3—Body fat loss 4—Muscle mass loss 5—Fluid overload 6—Functional capability |
Reduces the impact of fluid retention Adds functional capacity Includes subjective variables |
Limited data in clinical practice. High sensitivity and specificity. |
2.2. Diagnosis of Malnutrition
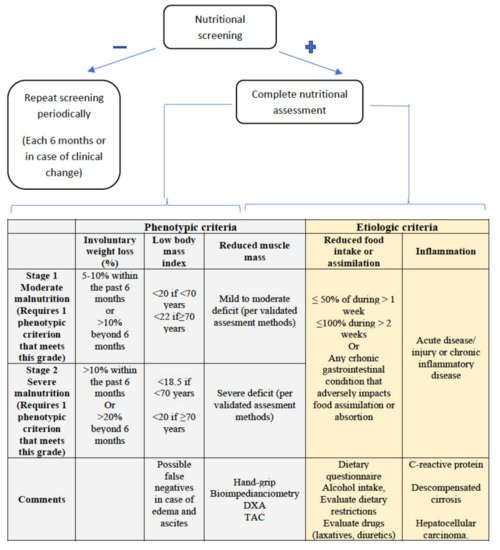
2.2.1. Assessment of Reduced Intake
2.2.2. Weight Loss and Body Mass Index
2.2.3. Muscle Mass and Body Composition
2.2.4. Disease Burden/Inflammation
3. Nutritional Intervention in Liver Disease
This entry is adapted from the peer-reviewed paper 10.3390/nu13051650
References
- Cheung, K.; Lee, S.S.; Raman, M. Prevalence and Mechanisms of Malnutrition in Patients with Advanced Liver Disease, and Nutrition Management Strategies. Clin. Gastroenterol. Hepatol. 2012, 10, 117–125.
- Kalaitzakis, E.; Bosaeus, I.; Öhman, L.; Björnsson, E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: Correlations with energy intake and resting energy expenditure. Am. J. Clin. Nutr. 2007, 85, 808–815.
- Meyer, F.; Bannert, K.; Wiese, M.; Esau, S.; Sautter, L.F.; Ehlers, L.; Aghdassi, A.A.; Metges, C.C.; Garbe, L.-A.; Jaster, R.; et al. Molecular Mechanism Contributing to Malnutrition and Sarcopenia in Patients with Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 5357.
- Chapman, B.; Sinclair, M.; Gow, P.J.; Testro, A.G. Malnutrition in cirrhosis: More food for thought. World J. Hepatol. 2020, 12, 883–896.
- Stirnimann, J.; Stirnimann, G. Nutritional Challenges in Patients with Advanced Liver Cirrhosis. J. Clin. Med. 2019, 8, 1926.
- Kamran, U.; Towey, J.; Khanna, A.; Chauhan, A.; Rajoriya, N.; Holt, A. Nutrition in alcohol-related liver disease: Physiopathology and management. World J. Gastroenterol. 2020, 26, 2916–2930.
- Wu, J.; Meng, Q.-H. Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J. Gastroenterol. 2020, 26, 4567–4578.
- Zaccherini, G.; Bernardi, M. The role and indications of albumin in advanced liver disease. Acta Gastroenterol. Belg. 2019, 82, 301–308.
- Petta, S.; Cammà, C.; Scazzone, C.; Tripodo, C.; Di Marco, V.; Bono, A.; Cabibi, D.; Licata, G.; Porcasi, R.; Marchesini, G.; et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2009, 51, 1158–1167.
- Chetri, K.; Choudhuri, G. Role of trace elements in hepatic encephalopathy: Zinc and manganese. Int. J. Gastroenterol. 2003, 22, 28–30.
- Hanai, T.; Shiraki, M.; Imai, K.; Suetugu, A.; Takai, K.; Shimizu, M. Usefulness of Carnitine Supplementation for the Complications of Liver Cirrhosis. Nutrients 2020, 12, 1915.
- Schneeweiss, B.; Graninger, W.; Ferenci, P.; Eichinger, S.; Grimm, G.; Schneider, B.; Laggner, A.N.; Lenz, K.; Kleinberger, G. Energy metabolism in patients with acute and chronic liver disease. Hepatology 1990, 11, 387–393.
- Westerterp, K.R. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front. Physiol. 2013, 4, 90.
- Peng, S.; Plank, L.D.; McCall, J.L.; Gillanders, L.K.; McIlroy, K.; Gane, E.J. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: A comprehensive study. Am. J. Clin. Nutr. 2007, 85, 1257–1266.
- Augustyn, M.; Grys, I.; Kukla, M. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. Clin. Exp. Hepatol. 2019, 5, 1–10.
- Bajaj, J.S.; Khoruts, A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J. Hepatol. 2020, 72, 1003–1027.
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131.
- Ali, R.; Nagalli, S. Hyperammonemia; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021.
- Dasarathy, S.; Hatzoglou, M. Hyperammonemia and proteostasis in cirrhosis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 30–36.
- Guañabens, N.; Parés, A. Osteoporosis in chronic liver disease. Liver Int. 2018, 38, 776–785.
- Ii, D.Y.; Pan, K.; Shendge, V.B.; Liu, J.; Ebraheim, N.A. Linkage of microbiota and osteoporosis: A mini literature review. World J. Orthop. 2019, 10, 123–127.
- Lee, Y.-H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.-W.; Kang, E.S.; Cha, B.-S.; Han, K.-H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786.
- Meroni, M.; Longo, M.; Rustichelli, A.; Dongiovanni, P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int. J. Mol. Sci. 2020, 21, 2986.
- Longo, M.; Meroni, M.; Paolini, E.; Macchi, C.; Dongiovanni, P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): New perspectives for a fairy-tale ending? Metabolism 2021, 117, 154708.
- Longo, M.; Paolini, E.; Meroni, M.; Dongiovanni, P. Remodeling of Mitochondrial Plasticity: The Key Switch from NAFLD/NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4173.
- Loong, T.-W. Understanding sensitivity and specificity with the right side of the brain. BMJ 2003, 327, 716–719.
- Shaw, C.; Fleuret, C.; Pickard, J.M.; Mohammed, K.; Black, G.; Wedlake, L. Comparison of a novel, simple nutrition screening tool for adult oncology inpatients and the Malnutrition Screening Tool (MST) against the Patient-Generated Subjective Global Assessment (PG-SGA). Support Care Cancer 2014, 23, 47–54.
- McFarlane, M.; Hammond, C.; Roper, T.; Mukarati, J.; Ford, R.; Burrell, J.; Gordon, V.; Burch, N. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin. Nutr. ESPEN 2018, 23, 156–161.
- Yasutake, K.; Koga, S.; Hokko, Y.; Ikemoto, M.; Yaguchi, Y.; Sakai, H.; Murata, Y.; Ohe, K.; Kohjima, M.; Nakamuta, M.; et al. Relevance of the Mini Nutritional Assessment in cirrhotic liver disease patients. Asia Pac. J. Clin. Nutr. 2018, 27, 300–305.
- Boulhosa, R.S.S.B.; Lourenço, R.P.; Côrtes, D.M.; Oliveira, L.P.M.; Lyra, A.C.; De Jesus, R.P. Comparison between criteria for diagnosing malnutrition in patients with advanced chronic liver disease: GLIM group proposal versus different nutritional screening tools. J. Hum. Nutr. Diet. 2020, 33, 862–868.
- Harimoto, N.; Yoshizumi, T.; Inokuchi, S.; Itoh, S.; Adachi, E.; Ikeda, Y.; Uchiyama, H.; Utsunomiya, T.; Kajiyama, K.; Kimura, K.; et al. Prognostic Significance of Preoperative Controlling Nutritional Status (CONUT) Score in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma: A Multi-institutional Study. Ann. Surg. Oncol. 2018, 25, 3316–3323.
- Wang, T.; Shen, J. Usefulness of Simplified Nutritional Appetite Questionnaire (SNAQ) in Appetite Assessment in Elder Patients with Liver Cirrhosis. J. Nutr. Health Aging 2018, 22, 911–915.
- Georgiou, A.; Papatheodoridis, G.V.; Alexopoulou, A.; Deutsch, M.; Vlachogiannakos, I.; Ioannidou, P.; Papageorgiou, M.-V.; Papadopoulos, N.; Tsibouris, P.; Prapa, A.; et al. Evaluation of the effectiveness of eight screening tools in detecting risk of malnutrition in cirrhotic patients: The KIRRHOS study. Br. J. Nutr. 2019, 122, 1368–1376.
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9.
- Deza, D.C.; Msc, M.E.B.G.; Sanz-París, A.; Msc, M.L.B.; Bonilla, E.M.F.; Monterde, V.B.; Mainar, J.M.A.; Olmo, J.F. Mini Nutritional Assessment—Short Form Is a Useful Malnutrition Screening Tool in Patients with Liver Cirrhosis, Using the Global Leadership Initiative for Malnutrition Criteria as the Gold Standard. Nutr. Clin. Pract. 2021.
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329.
- Guerra, R.S.; Fonseca, I.; Sousa, A.S.; Jesus, A.; Pichel, F.; Amaral, T.F. ESPEN diagnostic criteria for malnutrition—A validation study in hospitalized patients. Clin. Nutr. 2017, 36, 1326–1332.
- Cichoż-Lach, H.; Michalak, A. A Comprehensive Review of Bioelectrical Impedance Analysis and Other Methods in the Assessment of Nutritional Status in Patients with Liver Cirrhosis. Gastroenterol. Res. Pract. 2017, 2017, 1–10.
- Sinclair, M.; Hoermann, R.; Peterson, A.; Testro, A.; Angus, P.W.; Hey, P.; Chapman, B.; Gow, P.J. Use of Dual X-ray Absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int. 2019, 39, 1089–1097.
- Buchard, B.; Boirie, Y.; Cassagnes, L.; Lamblin, G.; Coilly, A.; Abergel, A. Assessment of Malnutrition, Sarcopenia and Frailty in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients 2020, 12, 186.
- Belarmino, G.; Torrinhas, R.S.; Heymsfield, S.B.; Waitzberg, D.L. Sarcopenia in liver cirrhosis: The role of computed to-mography scan in the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Int. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1228.
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193.
- Bunchorntavakul, C.; Reddy, K.R. Review article: Malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment. Pharmacol. Ther. 2019, 51, 64–77.
- Iwasa, M.; Iwata, K.; Hara, N.; Hattori, A.; Ishidome, M.; Sekoguchi-Fujikawa, N.; Mifuji-Moroka, R.; Sugimoto, R.; Fujita, N.; Kobayashi, Y.; et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrients 2013, 29, 1418–1421.
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562.
- Tsien, C.D.; McCullough, A.J.; Dasarathy, S. Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 430–441.
- Chen, C.; Wang, L.; Kuo, H.; Fang, Y.; Lee, H. Significant effects of late evening snack on liver functions in patients with liver cirrhosis: A meta-analysis of randomized controlled trials. J. Gastroenterol. Hepatol. 2019, 34, 1143–1152.
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801.
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of Oral Branched-Chain Amino Acid Granules on Event-Free Survival in Patients with Liver Cirrhosis. Clin. Gastroenterol. Hepatol. 2005, 3, 705–713.
- Les, I.; Doval, E.; García-Martínez, R.; Planas, M.; Cárdenas, G.; Gómez, P.; Flavià, M.; Jacas, C.; Mínguez, B.; Vergara, M.; et al. Effects of Branched-Chain Amino Acids Supplementation in Patients with Cirrhosis and a Previous Episode of Hepatic Encephalopathy: A Randomized Study. Am. J. Gastroenterol. 2011, 106, 1081–1088.
- Fialla, A.D.; Israelsen, M.; Hamberg, O.; Krag, A.; Gluud, L.L. Nutritional therapy in cirrhosis or alcoholic hepatitis: A systematic review and meta-analysis. Liver Int. 2015, 35, 2072–2078.
- Koretz, R.L.; Avenell, A.; Lipman, T.O. Nutritional support for liver disease. Cochrane Database Syst. Rev. 2012, CD008344.
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017, 5, CD001939.
- Zenith, L.; Meena, N.; Ramadi, A.; Yavari, M.; Harvey, A.; Carbonneau, M.; Ma, M.; Abraldes, J.G.; Paterson, I.; Haykowsky, M.J.; et al. Eight Weeks of Exercise Training Increases Aerobic Capacity and Muscle Mass and Reduces Fatigue in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1920–1926.e2.
- Berzigotti, A.; Albillos, A.; Villanueva, C.; Genescá, J.; Ardevol, A.; Augustín, S.; Calleja, J.L.; Bañares, R.; García-Pagán, J.C.; Mesonero, F.; et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017, 65, 1293–1305.
- Tandon, P.; Ismond, K.P.; Riess, K.; Duarte-Rojo, A.; Al-Judaibi, B.; Dunn, M.A.; Holman, J.; Howes, N.; Haykowsky, M.J.F.; Josbeno, D.A.; et al. Exercise in cirrhosis: Translating evidence and experience to practice. J. Hepatol. 2018, 69, 1164–1177.
- Everhart, J.E.; Lok, A.S.; Kim, H.; Morgan, T.R.; Lindsay, K.L.; Chung, R.T.; Bonkovsky, H.L.; Ghany, M.G. Weight-Related Effects on Disease Progression in the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial. Gastroenterology 2009, 137, 549–557.
- Ericksen, R.E.; Lim, S.L.; McDonnell, E.; Shuen, W.H.; Vadiveloo, M.; White, P.J.; Ding, Z.; Kwok, R.; Lee, P.; Radda, G.K.; et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019, 29, 1151–1165.e6.
- Katagiri, R.; Song, M.; Zhang, X.; Lee, D.H.; Tabung, F.K.; Fuchs, C.S.; Meyerhardt, J.A.; Nishihara, R.; Chan, A.T.; Joshi, A.D.; et al. Dietary Intake of Branched-Chain Amino Acids and Risk of Colorectal Cancer. Cancer Prev. Res. 2019, 13, 65–72.
- Cabré, E.; Rodríguez-Iglesias, P.; Caballería, J.; Quer, J.C.; Sánchez-Lombraña, J.L.; Parés, A.; Papo, M.; Planas, R.; Gassull, M.A. Spanish Group for the Study of Alcoholic Hepatitis Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: A multicenter randomized trial. Hepatology 2000, 32, 36–42.
- Moreno, C.; Deltenre, P.; Senterre, C.; Louvet, A.; Gustot, T.; Bastens, B.; Hittelet, A.; Piquet, M.-A.; Laleman, W.; Orlent, H.; et al. Intensive Enteral Nutrition Is Ineffective for Patients with Severe Alcoholic Hepatitis Treated with Corticosteroids. Gastroenterology 2016, 150, 903–910.e8.
- Stokes, C.S.; Volmer, D.A.; Grünhage, F.; Lammert, F. Vitamin D in chronic liver disease. Liver Int. 2013, 33, 338–352.
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16.
- Takuma, Y.; Nouso, K.; Makino, Y.; Hayashi, M.; Takahashi, H. Clinical trial: Oral zinc in hepatic encephalopathy. Aliment. Pharmacol. Ther. 2010, 32, 1080–1090.
- Sinclair, M.; Grossmann, M.; Hoermann, R.; Angus, P.W.; Gow, P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J. Hepatol. 2016, 65, 906–913.
- Tsien, C.; Shah, S.N.; McCullough, A.J.; Dasarathy, S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur. J. Gastroenterol. Hepatol. 2013, 25, 85–93.
