Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
The surface-enhanced Raman scattering (SERS) technique, that uses magnetic plasmonic particles (MPPs), is an advanced SERS detection platform owing to the synergetic effects of the particles’ magnetic and plasmonic properties. As well as being an ultrasensitive and reliable SERS material, MPPs perform various functions, such as aiding in separation, drug delivery, and acting as a therapeutic material.
- Surface-Enhanced Raman Scattering (SERS)
- magnetic nanoparticles
- plasmonic nanoparticles
- detection
- drug delivery
- cancer therapy
- biological application
1. Introduction
In 1974, Fleischmann et al. first reported surface-enhanced Raman scattering (SERS) when they observed an unexpectedly strong Raman signal from pyridine adsorbed on a roughened silver electrode [1]. Since then, many studies have verified that the SERS phenomenon is related to electromagnetic and chemical effects [1,2]. Currently, SERS is a popular spectroscopic technique based on the plasmon-assisted scattering of molecules on or near metal nanostructures. It is widely used owing to several advantages, such as simple sample preparation, capability of detecting multiple analytes, and non-destructive analysis at a high sensitivity level [3,4,5,6,7,8,9,10,11].
Magnetic nanoparticles (MNPs) are 1–100 nm sized nanoparticles that can be manipulated using external magnetic fields [12,13]. Owing to their excellent physicochemical properties, studies on MNPs as optical probes or sensors have been undertaken in a wide range of fields, such as biotechnology/biomedicine [12,13,14,15,16]. However, bare MNPs possess high chemical activity; thus, they easily agglomerate or oxidize [12]. Therefore, surface functionalization of MNPs is required in many applications. Combining MNPs with other materials, such as plasmonic nanoparticles to create magnetic plasmonic particles (MPPs), can also help overcome these shortcomings.
Integrating MNPs into SERS has multiple benefits owing to the combination of plasmonic and magnetic properties, including high sensitivity, finger-print specificity, non-destructive detection of SERS characteristics, rapid separation, and simple external data gathering. Additionally, time-consuming complex matrix extraction of analytes is unnecessary [13,17,18].
2. Types of MPPs
Magnetic SERS requires materials possessing magnetic and plasmonic properties. Several kinds of MPPs have been designed and modified through the addition of functional molecules as per the requirement. This section explores the features and properties of a variety of MPPs.
In core-shell type MPPs, the magnetic and plasmonic parts are combined into one particle, typically having a magnetic core with a plasmonic shell structure. These are also known as combined-type MPPs. This type acts as a multifunctional material with both magnetic and plasmonic characteristics, such as moving under a magnetic field, and thus is a material potentially suitable for use in SERS. In contrast, separate-type MPPs have the magnetic and plasmonic parts in two distinct particles. The advantage of creating dual particles is that the magnetic particles, which can be manipulated by external magnetic fields, and the plasmonic particles facilitate SERS measurement.
Nanoparticles (NPs) can be labeled by surface modification with molecules like Raman label compounds or remain as label-free NPs. Generally, label-free NPs are used in their natural form without modification for direct detection of target molecules. The intensity of the intrinsic SERS spectrum depends on the target affinity of the NP surfaces and the target concentration [18,19]. Although label-free NPs can separate and detect targets using the intrinsic and specific SERS spectrum, an impure target can cause non-specific signals, which leads to difficulty in identification. Therefore, to ensure specific detection, NPs are modified with labeling molecules and bio-ligands—such as antibodies [18,20,21,22,23,24], aptamers [25,26,27], and polysaccharide (chitosan [28])—that bind with specific targets. Labeled MPPs can only recognize the target, and thus the detection accuracy increases.
MPPs are also classified according to their chemical composition: monometallic, alloyed, and assembly particles. The magnetic and plasmonic parts of monometallic MPPs are facilitated by a single type of metal, such as Fe3O4, Ag, or Au. This is a common type of MPPs [17,18,20]. Alloyed MPPs owe their magnetic and plasmonic parts to two or more metals, such as Co-Fe2O4 [29,30], Mn-Fe3O4 [25], Fe3O4-TiO2 [31], Fe2Ni [32], and Au-Ag NPs [22,25]. Their characteristics are strongly dependent on the structure and composition of the nanomaterials [33]. Alloy metal NPs are usually employed to obtain synergistic effects, such as optical tuning and enhanced stability, owing to their hybrid characteristics.
Assembly MPPs are MPPs combined with non-metals, such as polyethylenimine (PEI) [34,35,36,37], SiO2 [38,39,40], graphene oxide [32,41,42,43], and poly(N-isopropylacrylamide) (pNIPAM) [44]. PEI is a hydrophilic macromolecule from the primary amine group; therefore, it is introduced as an interlayer to significantly improve the dispersion and adsorption of negatively charged Au NPs [36,37]. When looking to identify bacteria in solution, PEI is introduced as a positively charged outer layer to promote strong electrostatic interaction with negatively charged bacterial pathogens [34]. In SERS, a silica shell has proven popular due to its advantageous properties, such as chemical inertness, transparency, hydrophilicity, and biocompatibility [45]. Silica encapsulation ensures the particles are well-dispersed in a solution, have long-term stability, and are suitable for surface modification via silane-coupling chemistry, thereby ensuring bioapplication [46,47]. Additionally, graphene and graphene derived materials, such as graphene oxide and reduced graphene oxide, are also promising substrates for SERS techniques [42]. These substrates are a monolayer of carbon atoms that undergo π–π stacking or electrostatic bonding with aromatic compounds. This greatly enhances the substrates’ ability to adsorb aromatic molecules [42,43,48]. Additionally, graphene has other benefits when used with metals in a hybridized form. A combination of graphene with noble metal nanostructures exhibits higher SERS enhancement [42], and graphene with magnetic metal has good electrical conductivity and stability when biomolecules immobilize onto surfaces [49]. Finally, graphene can reduce SERS noise.
Recently, MPPs have been designed with increasingly specific functions. For example, core-shell type MPPs with antibody labeling, have been fabricated from an antibody immobilized popcorn shaped magnetic core with gold shell particles. These MPPs have been used as a multifunctional nanomaterial in magnetic separation, SERS imaging, and photothermal destruction of bacteria [24]. The MPPs are made in a two-step process, as shown in Figure 1. First, Fe NPs are synthesized by mixing a metal precursor, stabilizer, and reducing agent—iron chloride (FeCl3), tri-sodium citrate (TSC), and sodium borohydride (NaBH4), respectively. Second, hydrogen tetrachloroaurate (HAuCl4⋅3 H2O) and CTAB act as a shape-templating surfactant to form the gold shape. When used, the amine group surface modifications on the MPPs conjugate with antibodies.
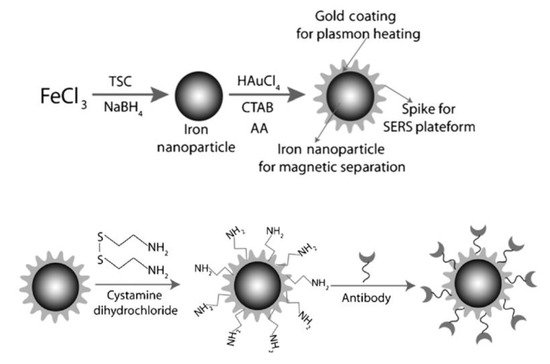
Figure 1. Popcorn shaped, magnetic core, gold shell nanoparticle synthesized and modified with an antibody. Reprinted with permission from ref [24]. Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In another example, Fe and Ag MPPs clusters [50] (Figure 2) are a combination of magnetic Fe3O4 NPs and plasmonic Ag NPs, and have been utilized for atrazine detection in pesticide polluted water. Through a solvothermal reaction, Ag NPs are formed by the decomposition of silver oleate. Then, smaller Fe3O4 NPs slowly diffuse and surround the surfaces of the Ag NPs, hence forming MPP clusters. Atrazine can be isolated, concentrated by the magnetic properties, and then detected by SERS. In a third example, a dual-particle style MPP, with labeling, is shown in Figure 3. These MPPs are formed when monodispersed silver-coated magnetic NPs are synthesized and conjugated with aptamer(1). The gold core and silver shell plasmonic nanoparticles, labeled with a SERS tag, 5,5′-dithiobis(2-nitrobenzoic acid); (DTNB), then conjugate with aptamer(2). The MPPs are applied for the specific identification of Staphylococcus aureus bacteria using SERS [25]. The bacteria are bonded by the magnetic NPs and separated by a magnetic bar, where SERS can then confirm the presence of the DTNB tag.
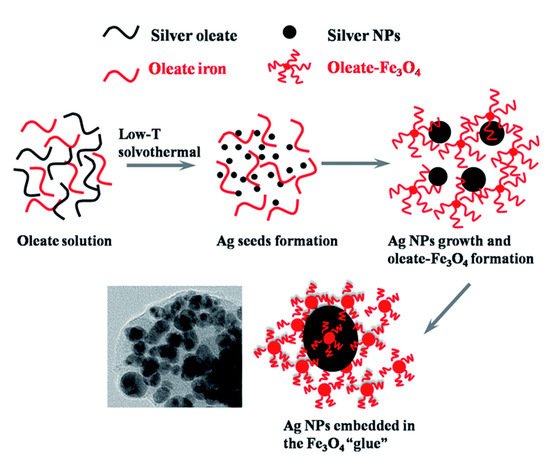
Figure 2. Magnetic Fe3O4 NPs and plasmonic Ag NPs forming an MPPs cluster. Reprinted with permission from ref [50]. Copyright © 2015 The Royal Society of Chemistry.
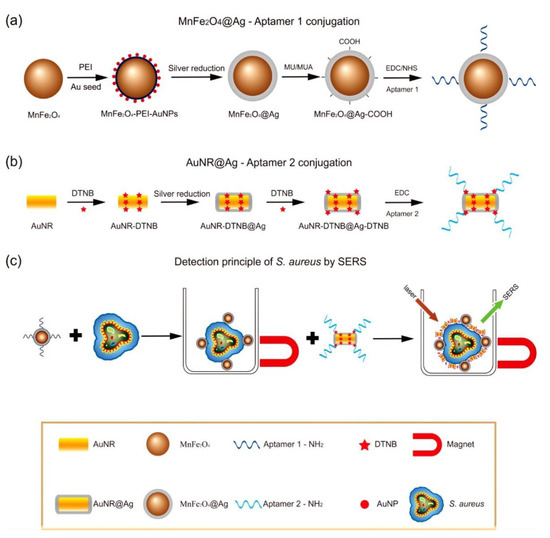
Figure 3. Scheme of Staphylococcus aureus detection by SERS using MPPs. (a) Synthesis of monodispersed silver-coated magnetic nanoparticles and their conjugation with aptamer 1; (b) Synthesis of gold core/silver shell plasmonic nanoparticles, labeled with SERS tag (DTNB), and their conjugation with aptamer 2; (c) operating principle for S. aureus detection. Reprinted with permission from ref [25]. Copyright © 2015 American Chemical Society.
3. Bioapplication of MPPs
Among various nanoparticles, plasmonic NPs and magnetic NPs are widely used in biological applications, but each have certain drawbacks that can be complimented by the other type of the NPs [51]. For example, the weakness of magnetic NPs such as toxicity [52,53], aggregation [54,55,56], easy corrosion in water [52] makes it difficult to work well in biological environments [52,53,54,55,56], while Au NPs hardly detect small amounts (giving low signal) of molecules in the mixture by SERS technology [57,58]. Once combined (e.g., in iron gold core- shell MPPs), the Au shell helps increase the biocompatibility of the hybrid, and the magnetic core helps increase the sensitivity in SERS detection of the hybrid. MPPs have characteristics of both magnetic and plasmonic metals. Therefore, they can be used as both SERS substrates and traditional magnetic NPs. Also, the interplay between the magnetic core and the plasmonic shell in the hybrids exhibits a synergistic effect in thermotherapy and bioimaging [21,51]. Some studies have reported on Fe-Au core shell MPPs, where the Au shell reduces the overall magnetic property, but this property is strong enough for MPP to act as a magnetic NP [21,35,51]. For example, in Han et al.’s study, the saturation magnetization of Fe3O4 NPs is 64.9 emu g−1; after the Au shell is coated, the saturation magnetization decreased to 59.2 emu g−1 and it still retains same coercivity (30 Oe) [21]; Zhou et al. showed the saturation magnetization after coating the Au shell of Fe3O4 decreased from 77.3 emu g−1 to 36.8 emu g−1 and 17.5 emu g−1 but maintained the same coercivity (306 Oe) [35]; both Fe3O4-Au core shell MPPs still retain magnetic property. On the other hand, the Au shell supplements better heating in magnetic hyperthermia [21] and Fe-Au core-shell MPPs exhibited a higher transverse relaxivity comparing with Fe3O4 NPs in bioimaging [51]. Another combination between magnetic and plasmonic is Ag and Fe based on MPPs that are similar with Au-Fe MPPs in sensitive specific detection [36,38,43,59], better bioimage [28] and moreover, they show antibacterial synergistic ability [60,61]. Padilla-Cruz et al. reported that core-shell Ag-Fe spherical particles showed magnetic and antimicrobial properties [60]. These Ag-Fe NPs possessed the antibacterial synergistic effect, compared to Ag NPs or Fe NPs, against both Gram-positive and Gram-negative multidrug-resistant bacteria and yeast. The Fe NPs have not showed the antibacterial property. Values of minimal inhibitory concentration of Ag NPs and Ag-Fe NPs are the same at 125 ppm in S.aureus ATCC 6538 case, 62.5 and 31.25 ppm in P. aeruginosa ATCC 27,853 case, 31.25 and 15.62 ppm in multidrug-resistant P. aeruginosa case, the same at 125 ppm in E. Coli ATCC 11,229 case, and 125 and 62.5 ppm in C. albicans case [60].
MPPs are applied in a wide range of settings. Many studies have reported MPPs with multiple functions in: targeted magnetic separation and enrichment, SERS imaging, and the photothermal destruction of bacteria [24]; cancer cell targeting, separation, and imaging [51,57]; free prostate specific antigen detection, MRI, and magnetic thermotherapy [21]. It further provides enormous advantages such as low cost [18,42,50,62], high sensitivity [18,27,42,62,63], good selectivity [18,27,42,62,63] and on-line monitor [50,51], and reproducibility [18,36]. For example, Wang et al. reported about using MPPs for SERS-based bacteria sensing, which has proved that MPPs are a low-cost material for bacterial detection with specific, sensitive advantages with the limitation of detection (LOD) for Staphylococcus aureus (S. Aureus) being 10 cells mL−1 [18]. In comparison with other methods like ELISA based on monodisperse magnetic particles (104–105 cells mL−1 LOD [64]), PCR method (10 cells mL−1 LOD [65]), real-time potentiometric biosensors based on carbon nanotubes and aptamers, (8 × 102 cells mL−1 LOD [66]), the sensitivity of MPPs based on SERS is better or equal but it is simpler in procedure while other approaches require special expensive instrumentation, trained technicians, or complicated pretreatment protocol [18]. Even in comparison with Au NP-based SERS (10–13 cells mL−1 LOD [67,68]), the method needs a separation step of target, MPP-based SERS only need 10 s for separation based on magnetic property [18]. For aromatic dye detection, SERS is a powerful method in this field; however, many aromatic dyes have a poor affinity in interactions with SERS material that leads to the necessity of modification of SERS substrate that can detect aromatic dyes as low as 1 nM LOD [69,70,71]. While this modification is often complex and high cost, one application of graphene oxide-wrapped MPPs as SERS substrate for aromatic dye detection exhibits the same sensitive specific detection as 1 nM LOD, and it is a lower cost material [42]. MPPs also show greater effectiveness than other approaches in protein detection. In platelet-derived growth factor BB detection, whereas MPPs based on SERS detect this protein as low as 0.1 pM LOD [62], other methods LODs are higher or similar, such as: 4 nM of Au NPs colorimetric sensor [72]; 80 pM of aptamer-based immunomagnetic electrochemiluminescence assay [73]; 68 pM of fluorescence [74]; and 0.5 pM of Au NPs based on SERS [75]. MPPs based on SERS has one more advantage which is quicker separation [62].
This entry is adapted from the peer-reviewed paper 10.3390/nano11051215
This entry is offline, you can click here to edit this entry!
