According to Encyclopedia Britannica, ion-exchange process can be defined as “any class of chemical reactions between two substances (each consisting of positively and negatively charged species called ions) that involves an exchange of one or more ionic components”.
This is the case, for example, of a multi-component oxide glass immersed – at a given temperature – in a mixture of molten salts containing metal ions (typically nitrates such as silver nitrate AgNO3, potassium nitrate KNO3, copper nitrate Cu(NO3)2, sodium nitrate NaNO3, etc.). Because of the high temperature at which the process occurs and concentration gradient established in proximity of the interface between glass and molten salt, sodium ions Na+ present within the compound glass migrate in the solution and are replaced by cations originally contained in the salt melt (e.g., Ag+, K+, Cu2+, etc.).
Due to the different size and polarizability of the ions participating in the process, the glass modifies its network locally in the exchanged regions, with particular reference to its density and, therefore, to its refractive index.
This paves the way for the production of graded-index optical components and waveguides, for passive and active integrated optical devices. Furthermore, the K+ – Na+ exchange is the basis for the chemical strengthening of the glass, which allows to obtain mechanically resistant glasses in increasingly thinner thicknesses for applications in smartphone technology and flexible photonics.
Finally, the possibility of inducing the formation of noble metal nanoparticles in an ion-exchanged glass following particular thermal post-process techniques allows the realization of new low-cost optical platforms for sensing and photovoltaic applications.
- ion-exchange
- optical glasses
- glass strengthening
- noble metal nanoparticles
- rare earths
- luminescence enhancement
- SERS
- flexible photonics
1. Introduction
To celebrate, over time, the wedding anniversary between ion-exchange and glass, all noble metals (e.g., silver, gold, platinum, etc.) and gemstones (e.g., sapphire, emerald, diamond, etc.) offered by our generous planet would not be enough! In fact, the origin of this lucky marriage is lost in the dawn of time. The art of ancient glassmakers can be traced back to the 3rd millennium BC among the peoples of Egypt and the Middle East where the main raw material, the silica rich sand, was widely available. From there, this knowledge was transferred to the peoples of the Mediterranean Sea, including the Greeks and the Romans (see, for instance, the natron glass used during the Roman Empire period). Over these three millennia, the colouring of glass was given directly during its manufacture by introducing additive metal oxides, such as iron, copper, manganese, etc., to the raw materials before firing the whole system in a kiln and then cooling it in order to obtain the final product [1,2].
Only between the 6th and 7th centuries AD, the Egyptians began to use silver or copper pigments—in the form of powders—to decorate and colour the surface of their artefacts, such as dishes, vessels and pots, by means of a thermal annealing process in a furnace. Through this technique, the pigment became part of the atomic structure of the products, colouring them in correspondence of their surface. However, due to the low temperature of the firing process and the difficulty of controlling it, the final artefacts remained rather opaque [3,4]. Later, around the 9th century AD, this staining method—improved in terms of technological process (i.e., higher operating temperature; better kiln performance) and now capable of giving glass lustre to ceramic artefacts—found great diffusion among the Mesopotamian peoples before arriving in Spain, in the first centuries of the millennium, following the expansion of Islamic culture [5,6]. In Europe, ion-exchange experienced a notable application during the outbreak of Gothic architecture (between 12th and 14th century) contributing to the realisation of the wonderful multi-coloured stained-glass windows of the cathedrals of that period. A paste, composed of clay/ochre and silver chloride/sulphide, was spread on the glass to be treated in order to produce a thin layer on its surface. Subsequently, the whole system was fired in a reducing atmosphere—induced in the furnace by introducing smoking substances—at a temperature close to the softening point of the glass. In these conditions, the ion-exchange process was triggered: the silver ions diffused inside the glass replacing the alkaline ions, such as sodium, widely present in the pristine glass. Finally, the simultaneous presence of reducing elements, both external (i.e., the smoking elements in the atmosphere) and internal to the glass (i.e., impurities, such as iron or arsenic), favoured the formation of silver metal nanoparticles (NPs) inside the ion-exchanged specimen, leading to a yellow-amber colour whose tonality and intensity depending on the process parameters (i.e., time and temperature) and the size of the NPs thus formed (from a few to one hundred nanometres), respectively. Other colours, such as red or green, were obtained using iron and copper salts, respectively. In the following renaissance period, this decorative technique became particularly popular in Italy in the creation of polychrome and lustre pottery artefacts, among which those of Deruta stood out par excellence [5,7].
It was only around the beginning of the 20th century that the foundations for a possible application of the ion-exchange process in the technical-industrial field were laid. In 1913 G. Schultze was the first to study the diffusion of silver ions into the glass using silver nitrate salt (AgNO3) as ion source, starting a whole series of studies aimed to understand the chemical-physical nature of the phenomenon and its effects on some physical properties of the glass so treated. In particular, a few years later, in 1918 at the Schott Glass Laboratory, it was demonstrated that ion-exchange produces an increase of the refractive index of the layer of the glass involved in the diffusive process [4,8]. Only from the 1960s, however, the ion-exchange technique became an industrial standard process.
Among all the possible applications, glass strengthening represented the first example of glass modification by this standardized technique. The process was initially investigated in 1962 by S. S. Kistler and P. Acloque (the latter in collaboration with J. Tochon) who, in their works, demonstrated how the in-diffusion of ions having different atomic size in the pristine glass matrix (i.e., larger potassium ions K+, originally present in the molten salt KNO3, in place of smaller sodium ions Na+ in the glass) was able to prevent or heal over the possible formation of micro/nano-cracks on the specimen surface, increasing its mechanical strength [9,10]. Since then, many efforts have been carried out until our days in this field both at research and industrial levels, and the development is still under way with amazing perspectives [10,11,12,13].
Almost simultaneously to glass strengthening, another key application of ion-exchange technique quickly began to establish itself. In fact, at the turn of ’60s and ’70s, the availability of the first optical fibres by Corning Inc (Corning, NY, USA), joined with the revolutionary vision of Bell Laboratories researchers J.E. Miller and colleagues, led to the aim of miniaturizing and integrating all necessary components for the generation, addressing, processing and detection of an optical signal on a single chip by means of optical waveguides and gave the decisive impulse for the development of integrated optics (IO). The ion-exchange process in glass greatly contributed to achieving these results [14,15,16,17]. Pioneering works in this field were given by T. Izawa and co-workers and T. G. Giallorenzi et al., at the beginning of the 1970s, who demonstrated the feasibility to obtain planar glass waveguides by thermal or field assisted ion-exchange using different molten salts, thus opening up the way for further development of novel integrated devices in the optical communications field [18,19]. Optical waveguides in glass by ion-exchange technique offered several advantages in comparison with other fabrication methods and materials: compatibility with optical fibres, low propagation and coupling losses, low birefringence, low cost in terms of material and fabrication process, high stability and reliability. All these features contributed in the following decades to the boom of IO devices with different functionalities, such as passive (e.g., for signal addressing and processing), active (e.g., for signal generation or amplification, thanks to rare earths doping), or even hybrid, when both functionalities coexist in a same chip [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
The third noteworthy application of ion-exchange was historically linked to the glass staining technique by the introduction of metal ions inside the pristine amorphous matrix during the process or, more frequently, by inducing their subsequent reduction into metallic form through an ad-hoc thermal post-process [40,41]. However, this approach of loading glass with metal ions or nanoparticles has been shown to go far beyond the mere colouring of the material. In fact, around the last decade of the 20th century, the progress in plasmonics—with the possibility to obtain both strong local field enhancement and high absorption cross-section by means of the surface plasmon resonance phenomenon in ion-exchanged dielectric substrates with embedded metal nanoparticles—has rapidly increased the research interest for these nanostructured materials [42,43,44,45,46,47], broadening their application horizon.
For instance, it was demonstrated that in ion-exchanged glasses the presence of noble metal nanoparticles (e.g., Au, Ag, or Cu), with their large third-order nonlinear susceptibility and ultrafast response, makes these materials suitable for applications also in non-linear optics [48,49,50,51,52,53].
Moreover, these embedded noble metal ions and nanoparticles played—and still do—an important role in the luminescence properties of the same ion-exchanged host materials especially when these are doped with elements belonging to the rare earth group, mitigating the limitation imposed on their concentration due to the occurrence of quenching phenomena. From the early works of Malta et al. [54] and Hayakawa et al. [55], the research literature on this topic has highlighted different reasons concerning the origin of the rare earth luminescence enhancement in optical glasses chemically treated by ion-exchange, depending on the nanostructure sizes so realized: (i) an energy transfer (ET) process between the energy levels of the noble metal ions (such as isolated Ag+, Ag+ - Ag+ pairs or silver aggregates) and those belonging to rare earths ions; (ii) an ET mechanism among non-plasmonic small metal nanoparticles (i.e., molecule-like nanoparticles having few nanometres size) and the doping active elements; (iii) a local field enhancement around the rare earth ions due to the surface plasmon resonance (SPR) phenomenon induced in small metal nanoparticles [54,55,56,57,58,59,60,61,62,63,64,65,66]. In the technological field, the aforementioned mechanisms—together with the down/up-conversion ones—have favoured the use of these ion-exchange glass systems in the photovoltaic sector in order to increase the solar cell efficiency through tailored cover-glasses and in the development of low cost and high performance white/coloured solid-state light sources [67,68,69,70,71,72,73,74,75,76,77,78,79,80,81].
On the other hand, in sensing/biosensing applications, low-loss ion-exchanged planar waveguides with embedded metal ions represented the core of integrated optical sensors for the analytical determination of biomolecules at the interfaces by an evanescent wave interrogation mechanism, exploiting different optical detection approaches such as those based on fluorescence or absorption [82,83,84,85,86,87,88,89,90]. Furthermore, these ion-exchange optical waveguides have been used as excitation systems for surface plasmon resonance (SPR) or localized surface plasmon resonance (LSPR) in noble metal thin films or NP arrays selectively deposited on them. This approach replaced the more traditional Kretschmann configuration based on prism coupling and contributed to the realisation of hybrid-plasmonic label-free integrated optical biosensors with high performance [91,92,93,94]. Alternatively, the same noble NPs can be directly grown in the guiding structure in order to obtain LSPR optical waveguides for the detection of selected analytes by monitoring the changes in absorption spectra as a result of the chemical reaction occurred at the interface [95]. Last but not least, the possibility of making optical waveguides in one-dimensional (1D) or two-dimensional (2D) geometry with embedded noble metal nanoparticles, starting from glass substrate by means of ion-exchange technique and an ad-hoc thermal post-process, has contributed in the last decades to the strong development of planar platforms for sensing/biosensing applications based on surface enhanced raman scattering (SERS) mechanism [96,97,98,99,100,101,102,103,104]. In comparison to the chemical procedures commonly adopted to bind metal NPs to the surface of a sample, the ion-exchange technique followed by a suitable thermal post-process represents a simpler and less expensive method for the manufacture of SERS substrates and provides the not negligible advantage of long-term stability of the metal nanoparticles thanks to their embedding within the same glass specimen [105,106,107].
2. Glass Strengthening: The Ion-Exchange Contribution
Today, it has become a cliché to associate the idea of glass with that of brittleness, so that, in the course of daily speaking, it is common to use the expression “fragile as glass” whenever one refers to an object destined to easily break. In this regard, Tennessee Williams, one of the most popular American playwrights, expresses himself in these terms about glass: “When you look at a piece of delicately spun glass you think of two things: how beautiful it is and how easily it can be broken” [108]. Moreover, the idea of brittleness associated with glass is so rooted in our mind that it stands as symbol of the uncertainty of life and the human condition as expressed by G. K. Chesterton in one of his aphorisms: “I felt and feel that life itself is as bright as the diamond, but as brittle as the window-pane” [109].
Yet, in spite of these beliefs, a pristine glass can tolerate high stresses, of the order of GPa. This makes glass an extremely strong material in itself [11,12,13,110]. A classic example is given by an optical fibre which, despite its size as thin as a hair, can achieve extraordinary mechanical tensile strengths (~few GPa) [111].
So, what does determine glass brittleness? The answer lies in the presence on its surface of microscale flaws occurring during the different steps of its manufacturing, processing and handling. Consequently, glass strength is drastically reduced by several orders of magnitude, down to only a few tens of MPa before reaching breaking point, once a mechanical stress is applied to its surface. If fused silica glass, thanks to its intrinsic structure, presents the best characteristics in terms of hardness and mechanical strength, multicomponent oxide glasses, such as soda-lime or float ones, are among the most produced due to their low cost and easier fabrication. However, due to their highly heterogeneous composition, they are more liable to the formation of surface defects during their processing and, therefore, more prone to breakage once they are subjected to mechanical stress. Unlike crystals which have a well-ordered internal atomic structure, glasses are characterized by a disordered and chaotic molecular configuration typical of the amorphous materials. The absence of a crystal lattice leads to the formation of shear bands in the material. From the observation of these bands that occurred in a metallic glass following their manufacturing process, A. Wisitsorasak and P. Wolynes developed a general theory capable of explaining how the breaking phenomenon occurs in these materials [112]. The model provides a mapping of all the possible configurations of the molecules in the solid, in order to describe how the mechanical stress changes the atomic rearrangement rate in the glassy material, with the consequent formation of the shear bands. The observation of these bands allows to identify the crystallisation points where the glass is structurally weaker and, therefore, could be more subject to breakage when exposed to external stress. The model can be extended and generalised for any other glass formulation. In this way it is possible to produce glass with better mechanical characteristics, which respond to different needs depending on their use. In addition to this promising theoretical model, currently, the main practical techniques for strengthening glass involve the use of thermal treatments or chemical tempering of the material [11,12,13].
2.1. Thermal Strengthening
Annealed, heat strengthened and chemically strengthened (or tempered) glass are the three main types of glass for general use. Annealed glass is the “regular” glass, as it comes from industrial glass manufacturing (float glass). Thermal strengthening involves a rapid cooling in air of the annealed glass surface after it has been heated to a temperature above the glass transition temperature Tg and close to the melting point of the material. At the beginning of this cooling process the glass surface cools down and contracts quickly, while its internal core remains hot in order to compensate dimensional changes with small relaxation stress. In this condition, the inner region of the glass is subject to compression while the external one is in tension. When the glass interior cools and contracts, the surfaces are already rigid and therefore residual tensile stresses are created inside the glass while compressive stresses occur at the surface. In comparison to a simple annealed glass, this thermal tempering process increases the strength of the pristine material because the applied stresses must overcome the residual compressive ones on the surface, thus preventing the breaking event [13,113,114]. This allows to increase the mechanical strength of the glass by about 4–5 times (up to a few hundred of MPa) and, at the same time, guarantees a better behaviour of the material in terms of safety when it is subject to a breakage [115]. In fact, when this event occurs, this kind of glass generally shatters into many small non-sharp and quite harmless fragments unlike what happens for annealed glasses which break in larger pieces, with a dangerous dagger-like profile. This particular response of tempered glass to a breakage event can be explained by the high strain energy present within these materials. The main limitation of the thermal strengthening process is linked to the minimum thickness obtainable for a tempered glass, which cannot reach values lower than a few millimetres (around 2–3 mm, typically) due to the rapid cooling process and the substantial difference in cooling rate from the glass core to its surface. This inevitably imposes further limits on the geometry and shape of the final products made with this type of glass, which must be simple and not very elaborate [11,12,13,67]. For all these reasons, thermally tempered glass finds its widest use in all those applications where human safety becomes an issue, such as in the automotive sector, for the development of side and rear windows in the vehicles, in the architectural one for the realisation of safety glass doors in private and public buildings, in the domestic environment (see, for instance, the glass for showers or microwave ovens), and the military/civil sector for the development of bulletproof glass where tempered glass is an essential component.
2.2. Chemical Strengthening
Another method to increase the strength of a multicomponent oxide glass involves modifying its chemical composition, replacing one of its constituent elements with another one that is different in terms of atomic dimensions and electronic polarizability and, at the same time, without significantly changing the network structure of the pristine glass. In particular, the replacement of small ions (originally present in the glass matrix) by larger ones (coming from an external ion source) produces a compressive stress at the surface of the glass for a “stuffing” or “crowding” effect, which can improve the glass strength by counteracting the surface flaws. This compressive stress at the surface is compensated by a tensile one in the internal region of the glass [7,11,12,13,110]. Generally, this method is applied at a temperature lower than the glass transition temperature Tg and involves the use of a mixture of molten salts as an ion source. For this reason, the “chemical strengthening” method is also referred to as “ion-exchange strengthening”. This technique prevents any risk of surface deformation and geometrical distortion due to the temperature parameter if compared to the thermal strengthening method. The resulting compressive stress so generated is generally higher than that achievable with the thermal tempering approach and it is confined in a thinner region of the glass, immediately below its surface, for low process temperature. In this case, the maximum compressive stress obtainable with this technique ranges from several hundreds of MPa to around 1 GPa in proximity of the glass surface [110,116]. Conversely, for a high process temperature (i.e., close to the glass Tg or above), the position of this maximum is located several microns below the glass surface and the magnitude of the stress rate decreases for the occurrence of surface stress relaxation due to viscous flow of the glass [13]. On the other hand, for a same process temperature, the maximum of the surface compressive stress decreases when the ion-exchange time increases: the depth at which the residual stress equals zero (i.e., the depth of layer, DoL) becomes deeper and, consequently, the flaws that can be involved in the process are larger [110,117,118]. On the other hand, the maximum for the tensile stress is not so easy to identify but, in some cases, it is located immediately below the compressive zone and its value is an order of magnitude lower than that of the compressive stress (~few tens of MPa), depending on the process duration and sample thickness [13]. Figure 1 compares the residual stress profile in the case of thermally and chemically toughened glass, respectively. Due to the stuffing effect of the incoming ions at the glass surface, the chemical strengthening method presents both large surface compression and internal tensile stress. The transition from the compression zone to the tension one is quite sharp. Conversely, the residual stress distribution in a thermal tempered glass takes on a parabolic profile depending on the temperature distribution achieved during the cooling step of the thermal tempering process. In this case, the compressive stress at the surface is approximatively twice the value of the tensile stress inside the specimen [11,12,13].
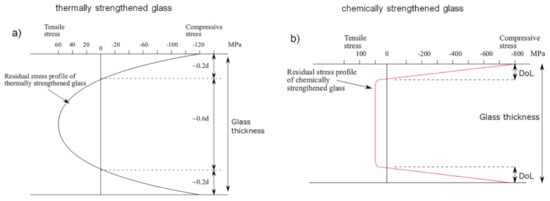
Figure 1. Residual stress profile through the glass thickness for: (a) thermally tempered glass; (b) chemically toughened glass. d is the glass thickness; DoL is the depth of layer. Figure reprinted from [67] under the terms of the Creative Commons Attribution 4.0 International License.
Several approaches have been proposed for stress evaluation in chemically toughened glasses. Generally, they are based on Cooper’s method which provides the determination of the stress in the glass starting from the knowledge of the concentration gradient for the ions participating in the exchange process [7,119]. In this context, R. Dugnani developed an analytical solution to the problem assuming a generalised function for the stress relaxation, a constant ionic inter-diffusion coefficient and, simultaneously, taking into account an analytical approximation for the composition-dependent stress relaxation behaviour of the glass [120]. On the other hand, A. K. Varshneya, G. Macrelli, and others in their studies modified the Cooper’s analysis introducing a new term in the model, related to different relaxation contributions (i.e., the viscoelastic and structural one) together with the network hydrostatic yield strength in order to evaluate the subsurface maximum compressive stress when the process temperature is higher and/or close to the glass transition temperature Tg [121,122,123,124]. Also noteworthy is the method, recently proposed by R. Rogoziński, which allows to control in real time the stresses generated in a glass as a result of the ion-exchange process from the knowledge of some parameters such as the elasto-optical coefficients, the dependence of the diffusion coefficients on the temperature and, finally, the function describing the time relaxation of stresses at the glass surface [118]. Differently from the numerical and analytical approaches mentioned above, N. Terakado and co-workers have developed a very original method for evaluating the compressive stress in a chemically strengthened glass, connecting it directly to the glass structure on an atomic scale. The non-contact and non-destructive method is based on the “stuffing” effect and the knowledge of three structural parameters, represented by specific boson peaks in the micro-Raman spectra of the sample [125].
At the end of this brief overview on the main concepts underlying the chemical strengthening of a glass, it should be remembered that in addition to the two most important process parameters, time and temperature, two other factors influence the formation of stress in a chemically strengthened glass: the composition of the pristine glass and that of the molten salt solution in which it is immersed. In fact, the larger the difference between the atomic dimensions of the two ionic species involved in the exchange, the greater is the magnitude of the compressive stress near the surface. In this sense, sodium-potassium (Na+ ↔ K+), lithium-sodium (Li+ ↔ Na+), or lithium-potassium (Li+ ↔ K+) exchanges are able to produce high compressive stresses with values that, practically, are close to 1 GPa in the best cases [11,12,13]. On the other hand, the composition of the pristine glass plays an important role on the diffusion rate of the ions and stress relaxation of the material during the exchange process. The common soda-lime glass has excellent diffusivity values for the incoming ions but a rapid relaxation process which makes difficult the achievement of a good glass strengthening despite its high alkali content [126]. However, the most promising glass formulation is the one that refers to aluminosilicates which have the highest diffusivity values while ensuring the achievement of compressive stresses well above the possible relaxation effects of the glass matrix [127,128]. In particular, the closer the alumina content is to that of the alkaline oxides, the higher the attainable strength of the glass following the exchange process [129]. For more details on the different characteristics of glass formulations, molten salt compositions, and ion-exchange typologies in glass strengthening, the reader is referred to the exhaustive reviews on the subject [11,12,13]. The main features of the two glass strengthening processes described above are compared in Table 1 below.
Table 1. A comparison between thermal and chemical glass strengthening methods.
| Parameter | Thermal Strengthening | Chemical Strengthening |
|---|---|---|
| Strength (max. value range) | ~(200–400) MPa | ~(800–1000) MPa |
| Surface compression layer | Thick | Thin |
| Stress distribution profile | Parabolic | More flat & square |
| Minimum sample thickness | 2–3 mm | <1 mm |
| Process Time | Short (mins) | Long (hours) |
| Process Cost | Cheap | Expensive |
| Glass Composition | No relevant | Alkaline glass (i.e., soda-lime or aluminosilicate) |
| Product Shape | Simple geometries | Unusual shape |
2.3. Chemical Strengthening: Applications
Although the chemical toughening process of glass presents expensive production costs due to its high time consuming if compared with a simple thermal tempering process, the possibility of obtaining greater mechanical strengthening in large glass surfaces with small thicknesses makes this process particularly suitable for many technological applications, ranging from the architectural (e.g., windows for buildings and palaces), automotive and transport sectors (e.g., windshield for airplanes and vehicles) up to the electronics and military ones (e.g., panels for displays in many electronic devices; air-to-ground missile launch tube protective covers). Figure 2 shows some outstanding applications related to chemical strengthened glasses.
Figure 2. Some relevant application fields of glass chemical strengthening.
In daily life, storing and sharing of data, from those personal to public ones, is almost delegated to electronic devices, such as smartphones, tablets, and lap-tops. The safety of these data does not only concern their protection at software level from any external attacks or a sudden failure of the operating system, but also requires the physical protection of the same devices, with particular reference to some of their most vulnerable elements such as monitor and/or display.
For this reason, CORNING® Inc., Asahi Glass Corporation (AGC), and SCHOTT AG, three world leaders in glass manufacturing, have developed in the recent years different high ion-exchange (HIE) tempered aluminosilicate glasses (for instance, the Gorilla® series from CORNING®, the Dragontrail™ from AGC, and the Xensation series from SCHOTT AG, respectively) with outstanding resistance to breakage and scratches in order to increase the protection for cover and touch screens, greatly reducing the risk of damage due to potential impact or fall and, consequently, avoiding any repair costs associated with this unpleasant event [130,131,132,133].
In all these cases, the characteristics of high surface resistance (>900 MPa in the best cases), flexibility (>700 MPa as a bending stress) and lightness (glass thickness < 1 mm, generally) are conferred to the pristine aluminosilicate glasses by the special chemical tempering treatment based on Na+ ↔ K+ ion-exchange, where the large K+ ions, substituting the host Na+ ions, force the generation of surface compression stress, supporting the closure of the cracks, thereby increasing the strength of the material and preventing the formation of others.
In this context, Apple, although employing Corning Gorilla glass technology to protect the displays of its smart devices, in recent years made a strong effort in the search of alternative solutions to further increase the conventional method of glass strengthening. Different strategies have been investigated, such as the implementation of a patterned asymmetric method based on double ion-exchange process in order to increase the compression depth over at least one localized region, with particular reference to the bend zones of the glass cover, as highlighted in some Apple patents recently published by the US Patent & Trademark Office [134,135,136]. Regarding the bulk material, the choice of the manufacturing company fell on a lithium aluminosilicate glass for its excellent chemical durability and mechanical resistance. The whole toughening process consists of two ion-exchange steps. In the first one, the specimen is immersed in a sodium nitrate NaNO3 salt (concentration range: 30–100% mol.) at a temperature lower than the Tg of the glass (process temperature range: 350–450 °C) for 4–5 h. In this case, the sodium ions, originally present in the melted salt, may exchange for smaller lithium ions in the glass matrix. This contributes to a first glass strengthening, allowing a build-up of sodium ions at the specimen surface. The second ion-exchange step, carried out in a potassium nitrate KNO3 salt (concentration range: 30–100% mol.) at a temperature lower than the glass Tg (process temperature range: 300–500 °C) for a process duration of 6–20 h, contributes to the definitive toughening of the glass. This double ion-exchange process was adopted by Apple both for the realisation of the front and rear glass cover of the iPhone11 smartphone generation. Moreover, the rear glass cover is also loaded with metal elements—through diffusion or deposition processes—in order to guarantee wireless charging operation for the final device.
Corning response to Apple arrived quickly with its Gorilla® Glass VictusTM, a chemically treated aluminosilicate glass capable of guarantee the best performance in terms of drop (up to 2 m) and scratch resistance among all the glasses belonging to the Gorilla® family. The Gorilla® Glass VictusTM presents a minimum thickness of about 0.4 mm and a shear modulus equal to 31.4 GPa, fully responding to the need for high mechanical strength in small thicknesses [137]. Due to these remarkable features, this cover glass was adopted by Samsung in some of its products such as, for instance, the Galaxy Note 20 Ultra.
Finally, it should be mentioned that the race for increasingly resistant materials is moving towards the use of glass-ceramics, characterized by the presence of nanocrystals within the amorphous glass matrix. In fact, a glass-ceramic is born like glass and then turns into an almost completely crystalline substrate by an ad-hoc thermal treatment able to induce the nanoparticle formation from additional nucleating agents, such as silver or titanium, previously introduced in the glass matrix. The dimensions of these nanostructures are generally of the order of a few nanometres, thus allowing an excellent transparency of the material. Moreover, glass-ceramics are tough, lightweight and present high temperature stability, high resistivity and excellent isolation capabilities. All these features are well suited to the role that these materials must assume in terms of safety of smart electronic systems. It is therefore no coincidence that, at the release of the new iPhone12 series in October 2020, Apple declared that it had realized—in collaboration with Corning—a new extremely resistant glass-ceramic product, the Ceramic Shield, four times better in terms of drop performance than Gorilla® Glass VictusTM [138]. Although Apple is not the first to have used glass-ceramic materials for the protection of its smart electronic devices (see, for instance, the Samsung with its smartphone Samsung Galaxy S10), the company from Cupertino is however the first to use such materials for the display protection while other manufacturing companies, such as Samsung, have used this material for back panel or camera-lens safety.
The chemical strengthening process of glass has recently found its application also in the automotive sector. In 2017, the American automotive manufacturer Ford was the first to equip its flagship automobile, the Ford GT supercar, using Gorilla glass for the windshield, rear window and engine cover of the vehicle, replacing the use of more traditional tempered glass. The new Gorilla hybrid glass, designed in strict collaboration with Corning, allowed a lightening of the overall weight of the automobile by well over 5 kg, increasing its manoeuvrability and reducing fuel consumption as well as the risk of glass breaking. Instead of using two thermally tempered sheets, joined together by a transparent thermoplastic adhesive in order to form the classic windshield glass, the new solution uses the co-presence of three layers: an external tempered glass joined to an internal one, highly resistant and chemically toughened, by a transparent and sound-absorbing thermoplastic. The presence of the ion-exchanged glass contributed to reduce the overall thickness of the new hybrid glass by approximately 50% compared to a common windscreen made with thermally tempered glass. Given the advantages of this strategy, it is likely that in the future the same technology will be extended to the production of series cars [139,140].
As a third significant example of ion-exchange application for glass strengthening, the case of photovoltaic and space solar cells deserves consideration. In fact, the cover glass represents one of the fundamental components of each of these devices and plays a key role in the final cost and efficiency of the cells. In terms of cost, the contribution relating to the protective glass is not negligible because it alone represents about 25% of the total cost of the whole device. Moreover, regarding the cell efficiency, the protective glass can influence the device performance depending on the transparency level of its material to solar radiation. Therefore, the optimisation of the cell cover glass becomes extremely important in order to minimize costs and maintain high performance for the final product.
In principle, glasses for photovoltaic and space solar cells should be as lightweight as possible in order to reduce material thickness and waste, so decreasing the overall cost of the device. Usually, commercial, thermally toughened float or soda-lime glasses, with a thickness of around 3 mm, are adopted on-board of the photovoltaic cells while they appear quite bulky and heavy for space solar cells. As a direct consequence of using a thin cover glass, comes the need for greater strength of the material. Moreover, the glass composition should be engineered for low losses and small UV photon absorption in order to guarantee better cell efficiency and service lifetime, respectively [67]. The use of chemically strengthened glasses may represent a valid solution, as reported by H. Wang et al. [141]. In that work, the authors demonstrated how an ion-exchange process, performed in a bath of pure KNO3 molten salt at a temperature comprised between 400 °C and 460 °C for a process time from 20 to 90 min, was able to confer suitable toughening to experimental silicate glasses having a thickness of only 120 μm. In particular, the maximum achievable flexural strength was measured to be around 632 MPa, four times higher than that of the un-treated samples. Moreover, the selected ionic process did not significantly modify the transparency characteristic of the pristine glasses in a wide range of the wavelength, while, at the same time, it was able to increase the durability of the material when exposed to the ion radiation. Hence, the obtained results were in full agreement with the possibility to reduce the cost and improve the performance of the space solar cell in terms of efficiency and service lifetime.
With the aim to release on the market a lighter glass, the researchers of the Asahi Glass Corporation (AGC) made a novel chemically-strengthened industrial glass, Leoflex™, an ultra-thin, flexible and extremely resistant material. Leoflex™ glass, with its lower sodium concentration at the surface, exhibits better electrical resistivity and higher mechanical strength caused by the chemical toughening process and strongly reduces the risk of potential induced degradation (PID), which represents one of the main reasons for the failure of photovoltaic and space solar cells. All these features made this material an ideal candidate as a cover glass for the development of highly performing photovoltaic application [142]. Additionally, the ion-exchange process also plays a key role in the efficiency of the same cell by introducing noble metal ions/nanoparticles capable of triggering particular energy transfer mechanisms (i.e., down-conversion from ultraviolet (UV) to visible wavelengths or up-conversion from infrared (IR) to visible range), especially when the glasses are doped with elements belonging to the rare earth group. This subject, together with other ones which involve the use of metal aggregates for different application fields, will be addressed in the next section.
This entry is adapted from the peer-reviewed paper 10.3390/app11104610
