Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
In the follicular lymphoma (FL) microenvironment, CXCR5+ICOS+PD1+BCL6+ T follicular helper (Tfh) cells, which closely correlate with FL B cells in neoplastic follicles, play a major role in supporting FL. Interleukin-4 secreted by Tfh cells triggers the upregulation of the lymphocyte chemoattractant CXCL12 in stromal cell precursors, in particular by fibroblastic reticular cells (FRCs).
- follicular lymphoma
- microenvironment
- follicular helper T cell (Tfh)
- fibroblastic reticular cell (FRC)
- follicular dendritic cell (FDC)
- HVEM/TNFRSF14
- T-follicular regulatory cell (TFR)
- idelalisib
- EZH2
- TIGIT
 1. Introduction
1. Introduction
Follicular lymphoma (FL) is the second most common B-cell non-Hodgkin lymphoma (B-NHL), and patients with FL commonly experience a slowly progressive disease. Given its indolent course, approximately 80% of patients already have advanced FL at the initial diagnosis. Patients with FL show a variable clinical path even without treatment, with spontaneous remissions being reported in up to 15% of the patients. Nevertheless, half of FL patients have histologically transformed diseases to aggressive lymphoma and finally succumb to the disease. A variety of treatment strategies for FL are currently used in the clinic, including observation without any treatment (so-called watchful waiting), rituximab alone, the anti-cluster of differentiation (CD)20 antibody combined with monotherapy or a combination of chemotherapy, radiotherapy in the case of limited disease, radioimmunotherapy (an anti-CD20 antibody conjugated with radioisotope), and high-dose chemotherapy followed by hematopoietic stem cell transplantation. Recently, the cumulative incidence of FL was reduced from 28–45% [1,2] to 9.3% at 10 years as a result of long-term observation following immunochemotherapy with the anti-CD20 antibody rituximab combined with chemotherapy [3].
Histopathology of FL is characterized by the formation of neoplastic follicles, which resemble the normal lymph node (LN) architecture. Many non-tumor cells surround the neoplastic cells in the FL LNs, some of which contribute to lymphomagenesis, including LN stromal cells, follicular helper T (Tfh) cells, follicular regulatory T cells (TFRs), and follicular dendritic cells (FDCs). The correlation between macrophage infiltration in FL LNs and patient prognosis remains controversial, but might vary according to the use of rituximab or different chemotherapeutic regimens [4]. In particular, a doxorubicin-containing regimen has been suggested to abolish the negative effect of CD163+ tumor-associated macrophages (TAMs) [4]. As for T cells, intratumoral T cells in FL LNs are heterogeneous depending on the prevalence of various T-cell subpopulations and location of the cells in relation to the follicles. For example, patients with intrafollicular or perifollicular (in a follicular pattern) forkhead box protein 3 (FoxP3)+ cells have a significantly higher risk of histologic transformation and shorter survival than those with FoxP3+ cells scattered in a diffuse pattern [5]. In contrast, high numbers of tumor-infiltrating FoxP3+ cells are related to improved overall survival (OS) in FL [6].
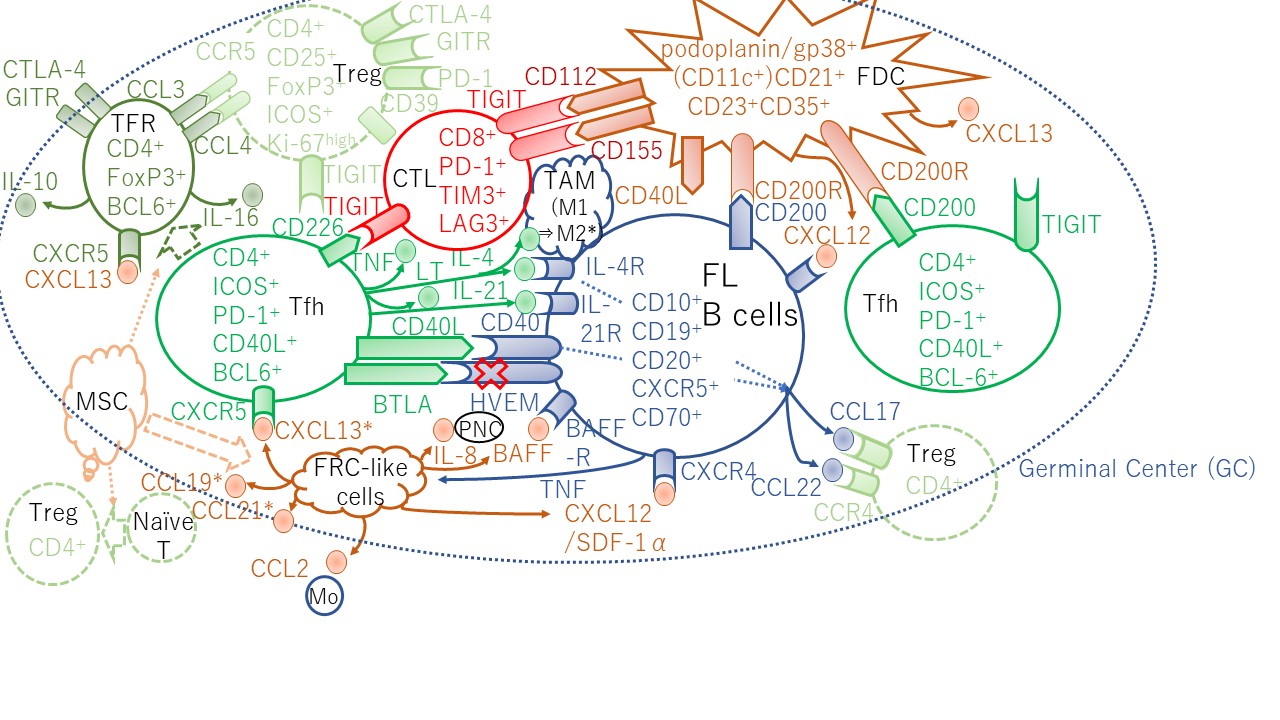
Programmed cell death-1 (PD-1) is a well-known T-cell exhaustion marker, but two distinct T-cell subpopulations with PD-1 expression were identified in FL. The intratumoral CD4+PD-1high T cells, which have a Tfh phenotype, express the C-X-C chemokine receptor type 5 (CXCR5), secrete interleukin (IL)-21, and are B-cell lymphoma 6 (BCL6) positive, but do not express the T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), and support B-cell growth. In turn, the CD4+PD-1low T cells, which have an exhausted phenotype, express TIM-3 and do not express either BCL6 or CXCR5 (Figure 1) [7]. Furthermore, T cells infiltrated in the FL LN are not simply exhausted, and there are other reasons contributing to their tolerogenic function.
Figure 1. Lymphoid stromal cells and CD4+ T cells supporting FL B-cell growth and allowing escape from immune surveillance with cytokine/chemokine circuits in the GC. The disruption of inhibitory signals delivered to the BTLA receptor increases T follicular helper (Tfh) cells. HVEM loss trigger production of TNF family cytokines that activate the lymphoid stroma cells; FDCs and FRCs. FRC-like cells produce CCL19 and CXCL13 that recruits CXCR5+ Tfh cells. In turn, Tfh cells produce IL-4 and IL-21 providing mitogenic signals to FL B cells. TFRs produce CCL3 (MIP-1α) and CCL4 (MIP-1β) are chemotactic for Tregs. * TAM polarization by IL-4 [8] and CXCL13, CCL19, and CCL21 secreted by normal FRCs [9] were results from mice experiments. In mice, TFRs originate from naïve Tregs [10,11,12]. In contrast, human FL TFRs in part originate from Tfh cells [13]. CD200R was found to be expressed just on classical DC (CD11c+HLA-DR+) from FL LNs. PMN, polymorphonuclear neutrophil; Mo, monocyte. Details in the text.
2. Mesenchymal Stem Cells Orchestrate the FL Cell Niche and Cancer-Associated Fibroblasts in the FL Microenvironment
Cancer-associated fibroblasts (CAFs) are phenotypically and functionally different from their normal counterparts, presenting a niche-based model of oncogenesis attributed to the dynamic coevolution of both cancer and stromal cells. Mesenchymal stem cells (MSCs) can be recruited within tumors, where they are integrated into the stroma, become activated, and augment tumor growth. LN containing bona fide MSCs, human bone marrow (BM)-MSCs, and LN-MSCs can be committed to FRC differentiation in response to a combination of TNF-α and lymphotoxin-α1β2 (LT), the two main factors implicated in the differentiation and sustenance of secondary lymphoid organs. Mesenchymal cells recruit malignant B cells and protect them from spontaneous and drug-induced cell death [49,50]. LT and TNF-α are two non-redundant key factors involved in lymphoma stromal cell differentiation and maintenance. B cells contribute to FRC activation and maintenance in both LN and spleen through their inducible expression of LT [32,51,52].
MSCs was shown to induce the differentiation of naïve T-cells to Tregs, thereby contributing for the modulation of the FL biology [53]. Thus, MSCs were proposed as organizers of the FL cell niche. MSCs overexpress CCL2, which recruit monocytes. Monocytes are further changed into proangiogenic and anti-inflammatory macrophages [54]. In particular, FL B cells can trigger the commitment of MSCs to differentiation into an FRC-like phenotype, and for MSCs to overexpress CCL2 and IL-8 in a TNF-dependent manner, resulting in FRC meshwork activation within involved LN and BM [49,54,55]. Furthermore, overexpression of IL-4 induces a transglutaminase highpodoplanin/gp38lowvascular cell adhesion molecule-1 (VCAM-1)highCXCL12high phenotype in human mesenchymal progenitors and FRC-like cells, a profile that resembles that identified in situ within the FL cell niche [56]. Additionally, TNF-α/LT and IL-4 induced the construction of an extracellular TG-positive meshwork. Whereas TNF-α/LT decreased TG mRNA, IL-4 induced both TG expression and redistribution at the surface of stromal cells [56]. Additionally, TNF-α/LT and IL-4 can induce the construction of an extracellular TG+ meshwork [56].
The upregulation of CXCL12 from FL-CAFs in FL-infiltrating LN and BM contributes to the migration, adhesion, and activation of FL B cells with a CXCR4+ phenotype [56] (Figure 1). BM obtained from FL patients with BM involvement revealed CD20+ FL B-cell aggregates with paratrabecular localization, which is characteristic of BM involvement of FL. These B-cell-infiltrated regions exhibited elevated CXCL12 expression compared with outside regions of FL infiltration [6]. FL B cells secrete TNF-α, previously involved in the induction of both CCL2 and IL-8 production by BM-MSCs [54,55]. Stromal cells cocultured with TNFhigh malignant B cells induced a decrease in CXCL12 expression. Conversely, purified CD4+CXCR5highPD-1high FL-Tfh cells induced elevation of CXCL12 in adipose tissue-derived stroma cells [56].
The specific downregulation of LT in FL B cells combined with upregulation of CXCL12 in FL-CAFs suggest a CRC-like origin of at least some FL stromal cells since dark zone CRCs, unlike FRCs and versatile cells, do not require LT and TNF-α to maintain CXCL12 expression and the network morphology [27]. Cultured FL BM stromal cells retained numerous features of their native counterparts, including the overexpression of CCL2, IL-8, and CXCL12, suggesting an imprinting of the stromal cells by the tumor context [54,55,56].
Autologous CAFs or the stromal cell line HS-5 were found to protect primary FL cells from apoptosis in response to the BCL2 inhibitor ABT-737 through mRNA induction of the adenosine triphosphate-binding cassette (ABC)-drug transporter genes ABC subfamily C member 1 (ABCC1) and ABC sub-family G member 2 (ABCG2), and upregulation of BCL2 like protein 1 (also known as BCL-XL) [57]. Furthermore, Sakamoto et al. demonstrated that pyruvate secreted from patient-derived CAFs supported the survival of primary FL cells [58].
(References would be added automatically after the entry is online)
This entry is adapted from the peer-reviewed paper 10.3390/ijms22105352
This entry is offline, you can click here to edit this entry!
