Cadmium (Cd) is an environmental toxicant with serious public health consequences due to its persistence within arable soils, and the ease with which it enters food chains and then, accumulates in human tissues to induce a broad range of adverse health effects. Diet is a primary exposure source for non-smoking populations, whilst cigarette smoke is an additional source of Cd among those who smoked. Cd exists in cigarette smoke as a non-volatile oxide form (CdO), and a volatile metallic form with high transmission rates. Of further concern, the electronegativity of Cd is similar to that of zinc (Zn), a nutritionally essential metal, whereas its ionic radius is similar to calcium (Ca) Thus, Cd can enter the body from the gut and lungs through the metal transporter systems and pathways evolved for acquisition and storage of Zn, Ca, and other nutritionally essential metals such as iron (Fe) and manganese (Mn).
- bladder cancer
- cadmium
- accquired cadmium tolerance
- Zinc homeostasis
- ZnT1 Zn(Cd) efflux transporter
- ZIP8 Zn(Cd) influx transporter
- ZIP14 Zn(Cd) influx transporter
1. Introduction
2. Cadmium-Induced Cell Transformation: An In Vitro Carcinogenicity Test
3. UROtsa Cell Line as a Cell Model to Dissect the Carcinogenicity of Cadmium
4. Zinc Transporters Expressed by Parental UROtsa Cells
| Zinc Transporters | Number of Transcripts in 1000 β-Actin | ||||
|---|---|---|---|---|---|
| Batch I, 0 µM Cd2+ | Batch II, 0 µM Cd2+ | 1 µM Cd2+ | 2 µM Cd2+ | 4 µM Cd2+ | |
| SLC30A family | |||||
| ZnT1 | 181 ± 23 | 365 ± 38 | 3007 ± 465 | 1434 ± 146 | 1216 ± 153 *** |
| ZnT2 | 0.01 ± 0.001 | 0.06 ± 0.01 | 73 ± 15 | 16 ± 1.9 | 11 ± 1.5 *** |
| ZnT3 | 0.03 ± 0.007 | 0.15 ± 0.01 | 0.24 ± 0.05 | 0.10 ± 0.02 | 0.10 ± 0.02 * |
| ZnT4 | 1.6 ± 0.26 | 11.4 ± 0.8 | 10 ± 1 | 8.7 ± 1 | 6.2 ± 0.5 ** |
| ZnT5 | 150 ± 19 | 510 ± 30 | 1038 ± 132 | 495 ± 54 | 568 ± 91 ** |
| ZnT6 | 4.5 ± 0.15 | 65 ± 8 | 77 ± 6 | 63 ± 13 | 57 ± 12 |
| ZnT7 | 734 ± 28 | 758 ± 76 | 1007 ± 136 | 706 ± 44 | 488 ± 63 * |
| ZnT10 | 0.04 ± 0.005 | 1.1 ± 0.2 | 2.4 ± 0.2 | 1.7 ± 0.2 | 1.1 ± 0.1 *** |
| SLC39A family | |||||
| ZIP1 | 19.5 ± 2.0 | 82 ± 9 | 99 ± 15 | 55 ± 10 | 59 ± 12 * |
| ZIP2 | 0.02 ± 0.004 | 1.2 ± 0.1 | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.2 ± 0.03 *** |
| ZIP3A | 9.1 ± 0.4 | 19 ± 1 | 23 ± 2.3 | 17 ± 1.7 | 14 ± 1.3 * |
| ZIP3B | 0.48 ± 0.05 | 4.1 ± 0.2 | 6.2 ± 0.7 | 4.4 ± 0.2 | 4.2 ± 0.4 * |
| ZIP4 | 0.18 ± 0.04 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 |
| ZIP5 | 0.01 ± 0.003 | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.002 |
| ZIP6 | 18.3 ± 2.6 | 92 ± 8 | 133 ± 12 | 80 ± 9 | 75 ± 10 ** |
| ZIP7 | 121 ± 9.5 | 204 ± 25 | 342 ± 69 | 149 ± 32 | 94 ± 21 *** |
| ZIP8 | 0.09 ± 0.01 | 2.1 ± 0.2 | 2.6 ± 0.3 | 2.0 ± 0.2 | 2.7 ± 0.4 |
| ZIP10 | 5 ± 0.3 | 54 ± 4 | 30 ± 8 | 14 ± 3 | 14 ± 3 *** |
| ZIP14 | 83.4 ± 10.5 | 146 ± 19 | 218 ± 24 | 158 ± 26 | 128 ± 19 * |
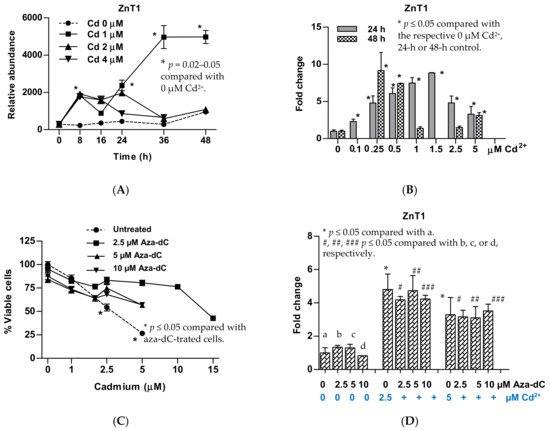
5. Upregulation of ZnT1 and Acquired Resistance to Cadmium
This entry is adapted from the peer-reviewed paper 10.3390/stresses1020009
References
- Pasin, E.; Josephson, D.Y.; Mitra, A.P.; Cote, R.J.; Stein, J.P. Superficial Bladder Cancer: An Update on Etiology, Molecular Development, Classification, and Natural History. Rev. Urol. 2008, 10, 31–43.
- Hong, Y.M.; Loughlin, K.R. Economic Impact of Tumor Markers in Bladder Cancer Surveillance. Urology 2008, 71, 131–135.
- Samanic, C.; Kogevinas, M.; Dosemeci, M.; Malats, N.; Real, F.X.; Garcia-Closas, M.; Real, F.X.; Garcia-Closas, M.; Serra, C.; Carrato, A.; et al. Smoking and bladder cancer in Spain: Effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1348–1354.
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445.
- Sciannameo, V.; Carta, A.; D’Errico, A.; Giraudo, M.T.; Fasanelli, F.; Arici, C.; Maule, M.; Carnà, P.; Destefanis, P.; Rolle, L.; et al. New insights on occupational exposure and bladder cancer risk: A pooled analysis of two Italian case-control studies. Int. Arch. Occup. Environ. Health 2018, 92, 347–359.
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood cadmium may be associated with bladder carcinogenesis: The Belgian case-control study on bladder cancer. Cancer Detect. Prev. 2007, 31, 77–82.
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated metallothionein-bound cadmium concentrations in urine from bladder carcinoma patients, investigated by size exclusion chromatography-inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2009, 631, 218–222.
- Achanzar, W.E.; Diwan, B.A.; Liu, J.; Quader, S.T.; Webber, M.M.; Waalkes, M.P. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001, 61, 455–458.
- Sens, D.A.; Park, S.; Gurel, V.; Sens, M.A.; Garrett, S.H.; Somji, S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol. Sci. 2004, 79, 56–63.
- Hoggarth, Z.E.; Osowski, D.B.; Freeberg, B.A.; Garrett, S.H.; Sens, D.A.; Sens, M.A.; Zhou, X.D.; Zhang, K.K.; Somji, S. The urothelial cell line UROtsa transformed by arsenite and cadmium display basal characteristics associated with muscle invasive urothelial cancers. PLoS ONE 2018, 13, e0207877.
- Benbrahim-Tallaa, L.; Tokar, E.J.; Diwan, B.A.; Dill, A.L.; Coppin, J.-F.; Waalkes, M.P. Cadmium Malignantly Transforms Normal Human Breast Epithelial Cells into a Basal-like Phenotype. Environ. Health Perspect. 2009, 117, 1847–1852.
- Soh, M.A.; Garrett, S.H.; Somji, S.; Dunlevy, J.R.; Zhou, X.D.; Sens, M.A.; Bathula, C.S.; Allen, C.; Sens, D.A. Arsenic, cadmium and neuron specific enolase (ENO2, γ-enolase) expression in breast cancer. Cancer Cell Int. 2011, 11, 41.
- Blommel, K.; Knudsen, C.S.; Wegner, K.; Shrestha, S.; Singhal, S.K.; Mehus, A.A.; Garrett, S.H.; Singhal, S.; Zhou, X.; Voels, B.; et al. Meta-analysis of gene expression profiling reveals novel basal gene signatures in MCF-10A cells transformed with cadmium. Oncotarget 2020, 11, 3601–3617.
- Jing, Y.; Liu, L.Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19.
- Qu, W.; Tokar, E.J.; Kim, A.J.; Bell, M.W.; Waalkes, M.P. Chronic Cadmium Exposure In Vitro Causes Acquisition of Multiple Tumor Cell Characteristics in Human Pancreatic Epithelial Cells. Environ. Health Perspect. 2012, 120, 1265–1271.
- Boonprasert, K.; Satarug, S.; Morais, C.; Gobe, G.C.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. The stress response of human proximal tubule cells to cadmium involves up-regulation of haemoxygenase 1 and metallothionein but not cytochrome P450 enzymes. Toxicol. Lett. 2016, 249, 5–14.
- Boonprasert, K.; Ruengweerayut, R.; Aunpad, R.; Satarug, S.; Na-Bangchang, K. Expression of metallothionein isoforms in peripheral blood leukocytes from Thai population residing in cadmium-contaminated areas. Environ. Toxicol. Pharmacol. 2012, 34, 935–940.
- Takiguchi, M.; Achanzar, W.E.; Qu, W.; Li, G.; Waalkes, M.P. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp. Cell Res. 2003, 286, 355–365.
- Qu, W.; Diwan, B.A.; Reece, J.M.; Bortner, C.D.; Pi, J.; Liu, J.; Waalkes, M.P. Cadmium-induced malignant transformation in rat liver cells: Role of aberrant oncogene expression and minimal role of oxidative stress. Int. J. Cancer 2005, 114, 346–355.
- Garrett, S.H.; Somji, S.; Sens, N.A.; Zhang, K.K. Prediction of the Number of Activated Genes in Multiple Independent Cd+2- and As+3-Induced Malignant Transformations of Human Urothelial Cells (UROtsa). PLoS ONE 2014, 9, e85614.
- Petzoldt, J.L.; Leigh, I.M.; Duffy, P.G.; Sexton, C.; Masters, J.R.W. Immortalisation of human urothelial cells. Urol. Res. 1995, 23, 377–380.
- Rossi, M.R.; Masters, J.R.W.; Park, S.; Todd, J.H.; Garrett, S.H.; Sens, M.A.; Somji, S.; Nath, J.; Sens, D.A. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ. Health Perspect. 2001, 109, 801–808.
- Sens, D.; Rossi, M.; Park, S.; Gurel, V.; Nath, J.; Garrett, S.; Sens, M.A.; Somji, S. Metallothionein isoform 1 and 2 gene expression in a human urothelial cell line (UROtsa) exposed to CdCl2 and NaAsO2. J. Toxicol. Environ. Health A 2003, 66, 2031–2046.
- McNeill, R.V.; Mason, A.S.; Hodson, M.E.; Catto, J.W.F.; Southgate, J. Specificity of the metallothionein-1 response by cadmium-exposed normal human urothelial cells. Int. J. Mol. Sci. 2019, 20, 1344.
- Sens, M.A.; Somji, S.; Lamm, D.L.; Garrett, S.H.; Slovinsky, F.; Todd, J.H.; Sens, D.A. Metallothionein isoform 3 as a potential biomarker for human bladder cancer. Environ. Health Perspect. 2000, 108, 413–418.
- Zhou, X.D.; Sens, M.A.; Garrett, S.H.; Somji, S.; Park, S.; Gurel, V.; Sens, D.A. Enhanced expression of metallothionein isoform 3 protein in tumor heterotransplants derived from As+3- and Cd+2-transformed human urothelial cells. Toxicol. Sci. 2006, 93, 322–330.
- Somji, S.; Garrett, S.H.; Toni, C.; Zhou, X.D.; Zheng, Y.; Ajjimaporn, A.; Sens, M.A.; Sens, D.A. Differences in the epigenetic regulation of MT-3 gene expression between parental and Cd+2 or As+3 transformed human urothelial cells. Cancer Cell Int. 2011, 11, 2.
- Satarug, S.; Garrett, S.; Somji, S.; Sens, M.; Sens, D. Zinc, Zinc Transporters, and Cadmium Cytotoxicity in a Cell Culture Model of Human Urothelium. Toxics 2021, 9, 94.
- Satarug, S.; Garrett, S.; Somji, S.; Sens, M.; Sens, D. Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium. Stresses 2021, 1, 78–89.
- Ajjimaporn, A.; Botsford, T.; Garrett, S.H.; Sens, M.A.; Zhou, X.D.; Dunlevy, J.R.; Sens, D.A.; Somji, S. ZIP8 expression in human proximal tubule cells, human urothelial cells transformed by Cd+2 and As+3 and in specimens of normal human urothelium and urothelial cancer. Cancer Cell Int. 2012, 12, 16.
- Audenet, F.; Attalla, K.; Sfakianos, J.P. The evolution of bladder cancer genomics: What have we learned and how can we use it? Urol. Oncol. Semin. Orig. Investig. 2018, 36, 313–320.
- Takeda, K.; Fujita, H.; Shibahara, S. Differential Control of the Metal-Mediated Activation of the Human Heme Oxygenase-1 and Metallothionein IIa Genes. Biochem. Biophys. Res. Commun. 1995, 207, 160–167.
- Nishito, Y.; Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697.
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 expression and function lead to impaired intracellular zinc homeostasis in cancer. Cell Death Discov. 2019, 5, 144.
- Ohana, E.; Sekler, I.; Kaisman, T.; Kahn, N.; Cove, J.; Silverman, W.F.; Amsterdam, A.; Hershfinkel, M. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J. Mol. Med. 2006, 84, 753–763.
