Vaccination, employing peptides, nucleic acids, and other molecules, or using pathogen-based strategies, in fact, is one of the most potent approaches in the management of viral diseases. However, the vaccine candidate requires protection from degradation and precise delivery to the target cells. This can be achieved by employing different types of drug and vaccine delivery strategies, among which, nanotechnology-based systems seem to be more promising.
- Corona
- COVID-19
- drug delivery
- lipid particles
- liposomes
- nanoliposomes
- SARS-CoV-2
1. Introduction
Viruses are microscopic infectious agents, which replicate only inside the living cells of host organisms. They infect all types of life forms from microorganisms (including archaea and bacteria) to plants, animals, and humans [1]. There are mainly four groups of viruses, namely: (i) bacteriophages (viruses that infect bacterial cells); (ii) plant viruses (e.g., TMV: tobacco mosaic virus); (iii) animal viruses (e.g., DHV: duck hepatitis virus; SIV: simian immunodeficiency virus); and (iv) human viruses (e.g., coronaviruses) (see Figure 1). Bacterial viruses, known as bacteriophages, are one class of viruses that are identified as the most widespread biological organisms on the earth [2][3]. They bind to bacterial cells and perfuse the viral genome into the cell via lytic or lysogenic cycles [4][5]. The genetic material of these types of viruses consists of either DNA or RNA molecules [6].
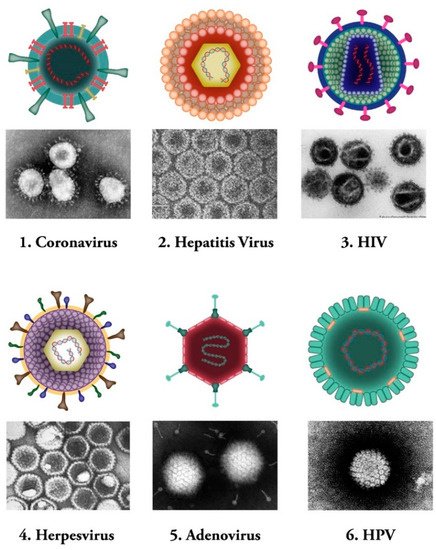
Figure 1. Common human viral pathogens. Six types of human infecting viruses, including coronavirus, hepatitis virus, human immunodeficiency virus (HIV), herpesvirus, adenovirus, human papilloma virus (HPV) are depicted schematically or as observed under electron microscopes.
Plant viruses, on the other hand, are parasitic organisms that use plant cells as their hosts. They rely on the host cellular resources and other factors essential for their replication cycle and movement [7]. Plant viruses utilize two types of movement, known as local (cell-to-cell movement in the infected leaves) and systemic (long-distance mobility through the plant vascular system). The genome of these viruses is made of ssDNA, dsRNA, positive-single-strand RNA, or negative-single-strand RNA [8][9].
Animal viruses are another group of viruses that cause infection and disease in animal cells. They need to identify a certain host cellular receptor for their entrance into the cell and the initiation of their life cycle [10]. The genome of animal viruses can be made of either DNA or RNA. Certain subgroups of animal viruses are classified as reverse transcribing viruses (also known as RT viruses), examples of which are simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) [11].
Human viruses, on the other hand, are defined as specific pathogens that have to confiscate some of the host cellular pathways to survive [12]. These viruses can either cause disease or could be asymptomatic [13][14]. Human viruses manifest a broad range of various structures [15]. Same as other groups of viruses mentioned above, Human viruses contain either RNA or DNA as their genome. Their nucleic acid material can be single- or double-stranded molecule. Human viruses are classified into several subgroups. Some of the human viruses, such as the herpes virus, might stay hidden in the body and be activated under specific circumstances of stress and immune system compromise [16]. Hepatitis B and C viruses are examples of another type of human pathogens that maintain constant existence and virulency in the body, and people are permanent carriers of these pathogens [17][18] (Figure 1).
2. Vaccination Platforms
Vaccines are medical tools that cause pathogen-specific immune responses in the body [19]. They induce the immune system to produce efficient antibodies that can attach to the antigens strongly and prevent them from infecting cells. In the recent outbreak of SARS-CoV-2, we are facing an urgent need for an efficient vaccine to minimize the threats of the virus on the human health and global economy as soon as possible [20]. Table 1 lists different types of vaccine protocols currently available to tackle viral diseases. Some of different available vaccine protocols are explained in the following sections.
Table 1. Different types of currently available protocols employed in vaccine construction.
| Vaccine Type | Target Pathogen/Disease | |
|---|---|---|
| Nucleic acid based vaccine | SARS-Cov-2 | |
| Peptide based vaccine | SARS-Cov-2, Hepatitis B | |
| Pathogen based vaccine | Live attenuated viral vaccine | Vaccina (smallpox), Measles, mumps, and rubella (MMR combined vaccine), Varicella (chickenpox), Influenza (nasal spray), Rotavirus, Zoster (shingles), Yellow fever |
| Inactivated viral vaccine | Polio (IPV), Hepatitis A, Rabies | |
| Viral subunit vaccine | Hepatitis B, Influenza (injection), Haemophilus influenzae type b (Hib), Pertussis (part of DTaP combined immunization), Pneumococcal, Meningococcal, Human papillomavirus (HPV) | |
| Adjuvant | Human papillomavirus (HPV) types 16 and 18, influenza (flu), Hepatitis B | |
2.1. Nucleic Acid-Based Strategies
The nucleic acid-based vaccines are generally selected from either an RNA molecule (e.g., mRNA) or a plasmid or a double-stranded DNA that encode antigens. These antigens provoke humoral and/or cell-mediated immune responses in the body [21][22]. The DNA/RNA -based vaccines are considered flexible, as they allow manipulating the antigens easily. The synthetic DNA/RNA stimulates the protein production, similar to the infection situation, in the target cells. The synthesized protein will be placed in the cytosolic plasma membrane (e.g., endoplasmic reticulum) and protein modification processes can precisely take place [23]. The important point towards this end is that DNA (or RNA) needs a delivery system to achieve its aims. In the context of nucleic acid-based COVID-19 vaccine formulation, liposomes and lipid nanoparticles (LNP), together with certain electrophysiological approaches (e.g., electroporation), in certain cases, appear to be among the most promising systems to deliver the nucleic acid construct to the target cell [24][25][26][27].
2.2. Peptide-Based Vaccines
Peptide-based vaccines have been designed against a variety of infectious and tumorigenic diseases [28]. Their design is based on the prediction and evaluation of specific epitopes of the B and T cells [29]. Epitopes are defined as antigenic regions, which are recognized by the immune cells and hold the immunogenic property for these cells [30]. To conduct an accurate cellular immune response in the body, antigenic segments should bind to a certain class of molecules known as Major Histocompatibility Complex (MHC). The antigenic fragments are processed by the MHC molecules, which will eventually present them to T cells. These peptidic epitopes should be linear in order to be able to bind MHC molecules. B cells possess receptors and are responsible for producing antibodies in the immune system [31]. Immunogenic carrier proteins, nanoparticle delivery systems, and potent adjuvants could be combined in the formulation in order to boost the immunogenicity of peptide-based vaccines. Several peptide-based vaccines (such as multi-epitope vaccines) against SARS-CoV-2 were formulated by interaction with T cells (CD4+ and CD8+) and B cells along with an adjuvant and assembling multi-epitope by using EAAAK linkers [32][33][34].
2.3. Pathogen-Based Strategies
2.3.1. Live Attenuated Viral Vaccines
Attenuated vaccines represent a weakened form of various pathogens, e.g., bacteria and viruses (including the yellow fever virus strain 17D) that lead to several different diseases. They induce potent and long-lasting cellular and humoral immunity in the body similar to the natural response, and are considered to be the oldest and the most efficient mode of vaccination [35][36][37]. Different approaches have been used for the production of the attenuated vaccines, such as employment of related non-human viruses (e.g., rotavirus, smallpox), administration at different body sites (adenovirus, influenza virus, herpes virus), and laboratory adaptation (polioviruses and picornaviruses) [38].
2.3.2. Inactivated Viral Vaccines
Whole virus vaccines utilize the complete particle of the virus that has been deactivated by processes employing certain chemical compounds, radiation, or heat. The immune responses of these type of vaccines qualitatively differ from those vaccines that are processed through intracellular pathways. In addition, inactivated viral vaccines often need extensive extra trials to confirm their safety because some studies have indicated increased infectivity following immunization [39][40][41].
2.3.3. Viral Subunit Vaccines
Subunit vaccines are specific antigenic segments (such as synthetic peptide or protein) of a pathogen, that are used to induce an immune response and provoke acquired immunity against the pathogen from which they are derived [42][43]. They possess high safety profiles, specifically targeting well-defined neutralizing epitopes [42][44]. There is no risk of incomplete inactivation, regain of virulence of the attenuated virus, or unfavorable host responses to viral subunit vectors [43][45]. Since the prevalence of SARS in 2002, subunit vaccines based on this virus have been studied and tested widely, demonstrating adequate efficacy and protection against SARS-CoV infections in several animal models [46][47][48][49].
2.4. Adjuvants
Adjuvants are pharmacological or immunological agents that are being used to improve immune responses. In other words, they are capable of increasing the biological half-life of vaccines and antigen uptake by the antigen-presenting cells (APCs). Currently, many adjuvants with different mechanisms of action are being utilized in vaccine constructs. Examples of adjuvants include mineral salts, saponins, cytokines, microbial components, microparticles, emulsions, and certain liposomes and nanoliposomes. They induce the production of immune regulatory cytokines, activate inflammations, local inflammation, cellular recruitment, and induce more rapid, broader, and stronger immune responses that are essential for good immunogenicity [50][51]. Nanoparticles and nanovesicles, due to their intrinsic adjuvanticity (by activating complement system, inducing autophagy and activation of inflammasome) are also considered as vaccine adjuvants nanosystems [52][53][54][55].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9050520
References
- Smith, A.E.; Helenius, A. How Viruses Enter Animal Cells. Science 2004, 304, 237–242. Available online: (accessed on 1 March 2021).
- Breitbart, M.; Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005, 13, 278–284.
- Martel, B.; Moineau, S. CRISPR-Cas: An Efficient Tool for Genome Engineering of Virulent Bacteriophages. Nucleic Acids Res. 2014, 2, 9504–9513. Available online: (accessed on 1 March 2021).
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage Resistance Mechanisms. Nat. Rev. Genet. 2010, 8, 317–327. Available online: (accessed on 1 March 2021).
- Vu, N.T.; Oh, C.S. Bacteriophage usage for bacterial disease management and diagnosis in plants. Plant Pathol. J. 2020, 36, 204–217. Available online: (accessed on 1 March 2021).
- Hatfull, G.F. Bacteriophage genomics. Curr. Opin. Microbiol. 2008, 11, 447–453.
- Garcia-Ruiz, H. Host factors against plant viruses. Mol. Plant Pathol. 2019, 20, 1588–1601.
- Garcia-Ruiz, H. Susceptibility Genes to Plant Viruses. Viruses 2018, 10, 484. Available online: (accessed on 1 March 2021).
- Plant Virology—Roger Hull—Google Books. Available online: (accessed on 1 March 2021).
- Avaratnarajah, C.; Warrier, R.; Kuhn, R. Assembly of Viruses: Enveloped Particles. In Encyclopedia of Virology; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; pp. 193–200.
- Molecular Virology of Human Pathogenic Viruses—1st Edition. Available online: (accessed on 1 March 2021).
- Greco, A. Involvement of the nucleolus in replication of human viruses. Rev. Med. Virol. 2009, 19, 201–214.
- Pride, D.T.; Salzman, J.; Haynes, M.; Rohwer, F.; Davis-Long, C.; White, R.A.; Loomer, P.; Armitage, G.C.; Relman, D.A. Evidence of a Robust Resident Bacteriophage Population Revealed through Analysis of the Human Salivary Virome. ISME J. 2011, 6, 915–926. Available online: (accessed on 1 March 2021).
- Wylie, K.M.; Weinstock, G.M.; Storch, G.A. Emerging view of the human virome. Transl. Res. 2012, 160, 283–290.
- Bushman, F.D.; McCormick, K.; Sherrill-Mix, S. Virus Structures Constrain Transmission Modes. Nat. Microbiol. 2019, 4, 1778–1780. Available online: (accessed on 1 March 2021).
- Chen, C.-H.; Chiu, Y.-L.; Wei, F.-C.; Koong, F.-J.; Liu, H.-C.; Shaw, C.-K.; Hwu, H.-G.; Hsiao, K.-J. High Seroprevalence of Borna Virus Infection in Schizophrenic Patients, Family Members and Mental Health Workers in Taiwan. Mol. Psychiatry 1999, 4, 33–38. Available online: (accessed on 1 March 2021).
- Lauer, G.M.; Walker, B.D. Hepatitis C Virus Infection. N. Engl. J. Med. 2001, 345, 41–52.
- Lee, W.M. Hepatitis B Virus Infection. N. Engl. J. Med. 1997, 337, 1733–1745.
- Pandolfi, F.; Franza, L.; Todi, L.; Carusi, V.; Centrone, M.; Buonomo, A.; Chini, R.; Newton, E.E.; Schiavino, D.; Nucera, E. The Importance of Complying with Vaccination Protocols in Developed Countries: “Anti-Vax” Hysteria and the Spread of Severe Preventable Diseases. Curr. Med. Chem. 2019, 25, 6070–6081.
- Ganti, R.S.; Chakraborty., A.K. Mechanisms underlying vaccination protocols that may optimally elicit broadly neutralizing antibodies against highly mutable pathogens. bioRxiv 2020, 330340.
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The new revolution in nucleic acid vaccines. Semin. Immunol. 2013, 25, 152–159.
- Piyush, R.; Rajarshi, K.; Chatterjee, A.; Khan, R.; Ray, S. Nucleic acid-based therapy for coronavirus disease 2019. Heliyon 2020, 6, e05007.
- Restifo, N.P.; Ying, H.; Hwang, L.; Leitner, W.W. The Promise of Nucleic Acid Vaccines. Gene Ther. 2000, 7, 89–92. Available online: (accessed on 1 March 2021).
- De Vrieze, J.; Louage, B.; Deswarte, K.; Zhong, Z.; De Coen, R.; Van Herck, S.; Nuhn, L.; Frich, C.K.; Zelikin, A.N.; Lienenklaus, S.; et al. Potent Lymphatic Translocation and Spatial Control Over Innate Immune Activation by Polymer–Lipid Amphiphile Conjugates of Small-Molecule TLR7/8 Agonists. Angew. Chem. Int. Ed. 2019, 58, 15390–15395.
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. Available online: (accessed on 1 March 2021).
- Bookstaver, M.L.; Tsai, S.J.; Bromberg, J.S.; Jewell, C.M. Improving Vaccine and Immunotherapy Design Using Biomaterials. Trends Immunol. 2018, 39, 135–150.
- Ye, T.; Zhong, Z.; García-Sastre, A.; Schotsaert, M.; De Geest, B.G. Current Status of COVID-19 (Pre)Clinical Vaccine Development. Angew. Chem. Int. Ed. 2020, 59, 18885–18897.
- Sabatino, D. Medicinal Chemistry and Methodological Advances in the Development of Peptide-Based Vaccines. J. Med. Chem. 2020, 63, 14184–14196.
- Hemmati, M.; Raoufi, E.; Fallahi, H. Predicting Candidate Epitopes on Ebola Virus for Possible Vaccine Development. In Advances in Ebola Control; InTech: London, UK, 2018; Available online: (accessed on 1 March 2021).
- Raoufi, E.; Hemmati, M.; Eftekhari, S.; Khaksaran, K.; Mahmodi, Z.; Farajollahi, M.M.; Mohsenzadegan, M. Epitope Prediction by Novel Immunoinformatics Approach: A State-of-the-art Review. Int. J. Pept. Res. Ther. 2020, 26, 1155–1163. Available online: (accessed on 1 March 2021).
- Raoufi, E.; Hemmati, M.; Einabadi, H.; Fallahi, H. Predicting candidate epitopes on Ebolaviruse for possible vaccine development. In Proceedings of the 2015 IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining 2015, Paris, France, 25–28 August 2015; Association for Computing Machinery, Inc.: New York, NY, USA, 2015; pp. 1083–1088.
- Lim, H.X.; Lim, J.; Jazayeri, S.D.; Poppema, S.; Poh, C.L. Development of multi-epitope peptide-based vaccines against SARS-CoV-2. Biomed. J. 2020, 44, 18–30.
- Kar, T.; Narsaria, U.; Basak, S.; Deb, D.; Castiglione, F.; Mueller, D.M.; Srivastava, A.P. A candidate multi-epitope vaccine against SARS-CoV-2. Sci. Rep. 2020, 10, 1–24.
- Kalita, P.; Padhi, A.K.; Zhang, K.Y.J.; Tripathi, T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 2020, 145, 104236.
- Weiss, C.M.; Liu, H.; Riemersma, K.K.; Ball, E.E.; Coffey, L.L. Engineering a fidelity-variant live-attenuated vaccine for chikungunya virus. Vaccines 2020, 5, 1–13.
- Salmona, M.; Gazaignes, S.; Mercier-Delarue, S.; Garnier, F.; Korimbocus, J.; De Verdière, N.C.; LeGoff, J.; Roques, P.; Simon, F.; Gazaigne, S. Molecular characterization of the 17D-204 yellow fever vaccine. Vaccine 2015, 33, 5432–5436.
- Vignuzzi, M.; Wendt, E.; Andino, R. Engineering Attenuated Virus Vaccines by Controlling Replication Fidelity. Nat. Med. 2008, 14, 154–161. Available online: (accessed on 1 March 2021).
- Powers, D.C.; Murphy, B.R.; Fries, L.F.; Adler, W.H.; Clements, M.L. Reduced Infectivity of Cold-Adapted Influenza A H1N1 Viruses in the Elderly: Correlation with Serum and Local Antibodies. J. Am. Geriatr. Soc. 1992, 40, 163–167.
- Chen, W.-H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 Vaccine Pipeline: An Overview. Curr. Trop. Med. Rep. 2020, 7, 61–64.
- Eckels, K.H.; Putnak, R. Formalin-inactivated Whole Virus and Recombinant Subunit Flavivirus Vaccines. In Advances in Virus Research; Academic Press Inc.: Cambridge, MA, USA, 2003; pp. 395–418.
- Jiang, S.; Bottazzi, M.E.; Du, L.; Lustigman, S.; Tseng, C.-T.K.; Curti, E.; Jones, K.; Zhan, B.; Hotez, P.J. Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rev. Vaccines 2012, 11, 1405–1413.
- Du, L.; He, Y.; Jiang, S.; Zheng, B.-J. Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today 2008, 44, 63–73.
- Deng, M.-P.; Hua-Lin, W.; Wang, H.-L.; Deng, F. Developments of subunit and VLP vaccines against influenza a virus. Virol. Sin. 2012, 27, 145–153.
- Zhang, N.; Jiang, S.; Du, L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev. Vaccines 2014, 13, 761–774.
- Zhang, N.; Channappanavar, R.; Ma, C.; Wang, L.; Tang, J.; Garron, T.; Tao, X.; Tasneem, S.; Lu, L.; Tseng, C.-T.K.; et al. Identification of an Ideal Adjuvant for Receptor-Binding Domain-Based Subunit Vaccines against Middle East Respiratory Syndrome Coronavirus. Cell. Mol. Immunol. 2016, 13, 180–190. Available online: (accessed on 1 March 2021).
- Wang, N.; Shang, J.; Jiang, S.; Du, L. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Front. Microbiol. 2020, 11, 29. Available online: (accessed on 1 March 2021).
- Du, L.; Zhao, G.; Chan, C.C.S.; Sun, S.; Chen, M.; Liu, Z.; Guo, H.; He, Y.; Zhou, Y.; Zheng, B.J.; et al. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology 2009, 393, 144–150.
- Zakhartchouk, A.N.; Sharon, C.; Satkunarajah, M.; Auperin, T.; Viswanathan, S.; Mutwiri, G.; Petric, M.; See, R.H.; Brunham, R.C.; Finlay, B.B.; et al. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: Implications for a subunit vaccine. Vaccine 2007, 25, 136–143.
- Zhang, N.; Tang, J.; Lu, L.; Jiang, S.; Du, L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015, 202, 151–159.
- Hajizade, A.; Ebrahimi, F.; Salmanian, A.H.; Arpanaei, A.; Amani, J. Nanoparticles in vaccine development. J. Appl. Biotechnol. Rep. 2014, 1, 125–134.
- Di Pasquale, A.; Preiss, S.; Silva, F.M.D.O.E.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. Available online: (accessed on 1 March 2021).
- Laupèze, B.; Hervé, C.; Di Pasquale, A.; Da Silva, F.T. Adjuvant Systems for vaccines: 13 years of post-licensure experience in diverse populations have progressed the way adjuvanted vaccine safety is investigated and understood. Vaccine 2019, 37, 5670–5680.
- Lakshmi, P.; Kumar, S.; Pawar, S.; Sudheesh, M.; Pawar, R.S. Plant-based Adjuvant in Vaccine Immunogenicity: A Review. Curr. Tradit. Med. 2018, 4, 215–236.
- Fries, C.N.; Curvino, E.J.; Chen, J.-L.; Permar, S.R.; Fouda, G.G.; Collier, J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat. Nanotechnol. 2020, 1–14.
- Garg, A.; Dewangan, H.K. Nanoparticles as Adjuvants in Vaccine Delivery. Crit. Rev. Ther. Drug Carrier Syst. 2020, 37, 183–204. Available online: (accessed on 1 March 2021).
