The advantages of metal organic frameworks (MOFs) are: existence of porosity to adsorb specific analyte, improved aqueous solubility, exceptional photophysical and chemical properties. MOFs are noted as exceptional candidates towards the detection and removal of specific analytes, particular for the detection/removal of environmental contaminants, such as heavy metal ions, toxic anions, hazardous gases, explosives, etc. Among heavy metal ions, mercury has been noted as a global hazard because of its high toxicity in the elemental (Hg0), divalent cationic (Hg2+), and methyl mercury (CH3Hg+) forms. To secure the environment and living organisms, many countries have imposed stringent regulations to monitor mercury at all costs. Regarding the detection/removal requirements of mercury, researchers have proposed and reported all kinds of MOFs-based luminescent/non-luminescent probes towards mercury.This review provides valuable information about the MOFs which have been engaged in detection and removal of elemental mercury and Hg2+ ions. Moreover, the involved mechanisms or adsorption isotherms related to sensors or removal studies are clarified for the readers. Finally, advantages and limitations of MOFs in mercury detection/removal are described together with future scopes.
- Hg2+
- CH3Hg+
- elemental mercury
- MOFs
- luminescent detection
- adsorption isotherms
- real analysis
- non-luminescent probes
- organic linkers
- metal nodes
1. Introduction
Due to the harmful and hazardous effects on ecosystem, detection/removal of mercury in different states, such as elemental, ionic, and organometallic (like methyl mercury), is in high demand and has attracted intense research interest [1,2,3,4,5]. Accumulated mercury in environmental water often sedimented and converted as toxic methylmercury, which entered the food cycle and caused serious diseases in living beings as stated next [6]. Accumulation of mercury in human body may lead to various health issues, such as brain damage, central nervous syndromes, Minamata disease, cognitive and motion disorders, etc. [7,8,9] Therefore, U.S. Environmental Protection Agency (EPA) regulated an allowable maximum level of mercury of 2 ppb (10 nM) in the drinking water and 3 ppm (1.5 μM) in fish tissue [10,11]. Moreover, Agency for Toxic Substances and Disease Registry (ATSDR) of the U. S. Department of Health and Human Service has set a highest allowable mercury concentration of 625 ppb in normal soil [1,2,3,4,5,6,7,8,9,10,11,12,13]. Till now the development of innovative tactics towards detection and removal of mercury are still the main focus of many research groups [12,13]. In this light, luminescent approaches comprising of nanoprobes, small molecules, supramolecular assemblies, aggregation induced emission, and covalent or metal organic frameworks (COFs/MOFs) are seemingly impressive with respect to their applicability, such as in vitro/vivo imaging studies [14,15,16,17,18,19]. However, design and development of metal organic frameworks (MOFs)-based probes towards specific analytes discrimination are highly anticipated with real time applications [20,21,21,23,24] due to the following advantages: existence of porosity to adsorb specific analyte, improved aqueous solubility, exceptional photophysical and chemical properties.In fact, the majority of the MOFs are composed of organic ligand and metal nodes with certain porosity and tend to form different micro/nano-structures, such as particles, cubes, rods, spheres, etc. [25,26,27,28,29] Moreover, they also find their applications in multiple opto-electronics, photovoltaics, electronics, solar cells, light emitting devices (LEDs), field effect transistors (FETs), DNA detection, bio-analysis, real time detection/removal of specific analytes, etc. [30,31,32,33] In sensory studies, they can behave as single, dual, and non-emissive materials, which tend to provide diverse responses upon interaction with guest analytes [34]. Moreover, the MOFs may display luminescent responses when interact with guest analytes via one-dimension (1D) (wavelength change and intensity alteration) or two-dimension (2D) (ratiometric variation combined with 1D responses) signals [21,35]. However, by tuning the functional organic units mediated responses, MOFs can be utilized in numerous sensory applications, such as pH sensors, virus and antibiotic detection, metal ions recognition, anions detection, volatile explosives quantification, etc. [36,37,38,39,40,41] Similarly, by modulating the functional units in MOFs, capture and removal of specific analytes can be achieved and become an effective approach for toxic analyte removal [42,43,44,45,46]. In this track, studies on detection and removal of highly toxic mercury and its analogous can find inspiring and exceptional applicability towards the environmental and health safety [47].So far, diverse MOFs have been demonstrated for exceptional detection and removal elemental mercury and Hg2+ [48,49,50,51]. In fact, detection of Hg2+ can be attained by assorted mechanisms, such as bands overlapping, ligand interaction, cation exchange, and framework collapse, etc. [20,33] On the contrary, removal of elemental mercury and Hg2+ ions are mostly achieved by tuning structural functionalities with organic ligands [52]. In addition, metal-organic coordination polymers and MOFs composited materials were demonstrated for mercury determination and removal [53,54]. So far, reviews on MOFs-based mercury detection and removal are deficient in valuable information, which encourages us to deliver a compact review to summarize the recent studies on this subject.In this review, we described the sensory detection and removal utilities of simple and polymeric MOFs and composited MOFs towards elemental mercury and Hg2+ as noted in Figure 1. Moreover, mechanism, electron transport, and structural benefits for mercury quantification were outlined for reader’s clarifications. Finally, a brief note on the synthesis of MOFs involved in discrimination and removal of mercury and its analogous was provided in this paper.
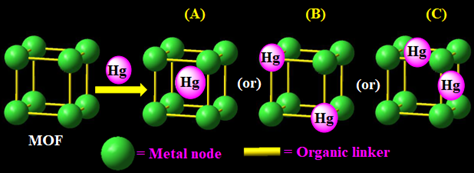
Figure 1. Schematic illustration of three possible MOFs-based mercury detection and removal mechanisms by (A) cavity trapping, (B) metal node displacement, and (C) interactive organic linkers.
2. Optimization Requirements for MOFs-Based Mercury Detection/Removal
- A.
-
Selection of suitable organic linkers that can form MOFs with designated metal nodes with greater surface area to interact with mercury analogous in the environment. Similarly, organic linkers with side chains, such as thiol (-SH; which shows the greater affinity to Hg2+, Hg0 and CH3Hg+), can be chosen for effective removal and detection mercury [1].
- B.
-
Selection of suitable metal nodes that can form MOFs with good stability in aqua/non-aqua solvents and afford large surface area for analyte (Hg2+) adsorption or collision for improving signal detection or quantification [2].
- C.
-
Selection of appropriate synthetic tactics/conditions to afford high yield over impurities. Similarly, the selection of suitable solvent for sensory studies also needs attention [3].
- D.
-
Many MOFs are well known coordinating polymers with porous nano/micro-structures [4], the design for capturing mercury requires more attention with respect to linkers, nodes, adsorbing ability, and opto-electronic properties.
- E.
-
In the case of removal of mercury from environmental samples, design and development of MOFs with high adsorption efficiency and stability in aqua medium need more attention and optimization for improvement [5].
- F.
-
To avoid the interfering effect from competing species, a unique MOF design with selectivity only to the mercury analogous must require optimization either by modulation of organic linkers or metal nodes or by tuning the opto-electronic properties [6].
- G.
-
Post-modification of MOFs with certain materials to form composited structures towards mercury detection/removal also requires optimization for authorized applicability [7].2. Optimization Requirements for MOFs-Based Mercury Detection/Removal
3. Synthetic Tactics Involved in MOFs Construction
By bridging the organic linkers with metal nodes, MOFs can be synthesized by many tactics as presented below [60].
- Diffusion method: This is a tactic that involves gradual conveyance of various species into interaction and can be sub-divided into (i) solvent liquid diffusion method, which takes place between precipitant solvent and product in the solvent and leads to crystallization at interface via gradual diffusion; (ii) gradual diffusion of reactants by adjusting the physical barriers, such as placing two reactant vials with different sizes to form MOFs [60,61].
- Hydro/solvothermal method: This technique involves self-assembly of products from soluble precursors. Wherein, precursors are introduced into the sealed tube under certain pressure and kept at 80–260 °C for days or weeks to produce the designated MOFs [60,62].
- Microwave method: In this tactic, solution containing small metal oxide particles is treated with microwave to raise temperature so that nano-sized metal crystals can be generated and leads to MOFs formation with controlled shape and size [63]. Contrary to other synthetic methods, microwave technology is a promising tactic with reduced reaction time and less processing energy consumption to have control over MOF properties. It is able to easily produce MOFs and MOF-hybrids in an isolated manner [64]. For example, Le et al. developed the mesoporous MOF-MIL-100 (Fe) via microwave-assisted continuous flow synthesis [by reacting iron(III) chloride hexahydrate (FeCl3⋅6H2O), 1,3,5-benzenetricarboxylic acid (H3BTC)] to support the construction of Cu(I) modified adsorbents towards CO/CO2 separation [65].
- Electrochemical method: This tactic is generally used in the industry to produce MOFs in bulk. Contrary to solvothermal synthesis, this method has the advantage of quick synthesis at low temperature and also avoids usage of anionic metal salts, such as metallic nitrates [66]. However, fine tuning in applied voltage is required to attain better results towards designed MOFs.
- Mechanochemical method: Contrast to traditional way of synthesis (dissolving, heating, and stirring chemicals in a solution), this method is environmentally friendly for synthesizing MOFs via mechanical forces, such as grinding and ball milling at ambient temperature without any solvent consumption. Moreover, certain number of MOFs can be obtained in a short time (10–20 min). This method is also noted as a technique at the interface of mechanical engineering and chemistry [67].
- Sonochemistry method: This is a quick synthesis tactic reported for producing MOFs in an environmentally friendly manner via treating the reaction mixture with high energy ultrasound force (10–20 MHz with upper limit of human hearing). During this process, dissolution of the starting materials can be enhanced, thereby becoming a special research topic for scientists for producing MOFs in bulk [68].
- Post-synthetic modifications: Apart from the aforementioned tactics, the designated MOFs can be synthesized via post-synthetic modifications, such as ligand exchange, metal exchange, opening of the coordinating sites, etc. [69]
4. MOFs in Optical Detection of Hg2+
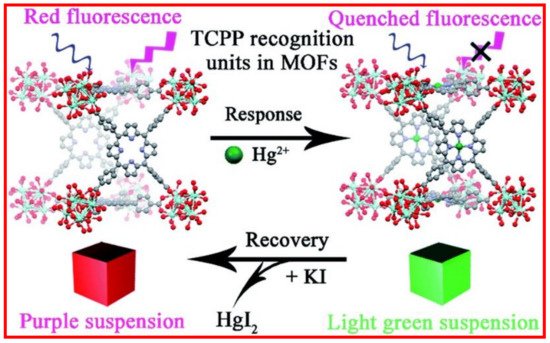
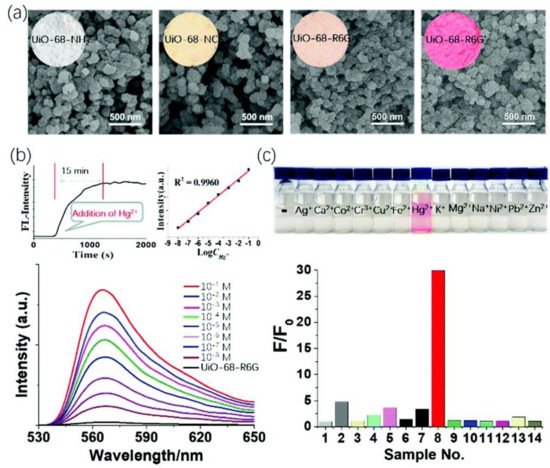
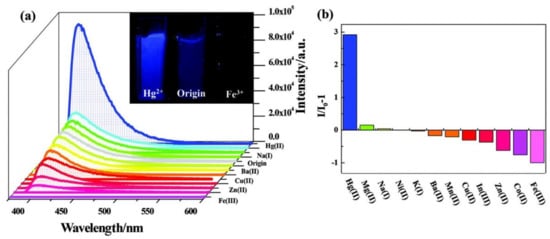
5. Metal Coordinated Polymers as Luminescent Hg2+ Sensors
In addition to MOFs, metal containing coordination polymers were proposed towards luminescent sensing of Hg2+ [100]. For instance, Sun’s research group developed Zn- and Cd-based coordination polymers-[Zn(TPDC-2CH3)(H2O)2].H2O and [Cd(TPDC-2CH3)(H2O)4].H2O via solvothermally reacting 2′,5′-dimethyl-[1,1′:4′,1′′-terphenyl]-4,4′′-dicarboxylic acid (H2TPDC-2CH3) with Zn2+ and Cd2+ ions, separately, and engaged them in sensory interrogations towards metal ions [102]. Emission of the [Zn(TPDC-2CH3)(H2O)2].H2O metal polymer at 380 nm (in water) was linearly quenched between 1–10 femtomole (fM) with a LOD of 3.6 fM. The solid chelation-enhanced fluorescence quenching (CHEQ) effect can be attributed to coordination between carboxyl group and Hg2+. However, this report did not provide any information regarding the BET surface area and practicality. Next, the Eu/IPA CPNPs (by solvothermal tactic) were prepared by reacting Eu3+ comprising coordination polymer nanoparticles (CPNPs) with isophthalic acid (IPA) bridging ligands and were employed in Hg2+ detection [103]. Initially, absorbance band of Eu/IPA CPNPs in Tris–HCl buffer (25 mM, pH 7.0) was overlapped with imidazole-4,5-dicarboxylic acid (Im), thus emission intensity at 615 nm was quenched due to the inner filter effect (IFE). During addition of Hg2+, the IFE was supressed and recovery of emission at 615 nm was observed. The linear correlation of Hg2+ ranged between 2 nM to 2µM with a LOD of 2 nM. Effectiveness of the probe was further demonstrated with applicability in biological fluid samples. Nevertheless, information regarding BET surface area must be evaluated for its exceptional applicability.
Towards the development of metal coordination polymers for Hg2+ sensors, Li et al. described the solvothermally synthesized Zn-based 3D coordination polymer-{[Zn2(1,4-bpyvna)(1,3,5-BTC)(OH)]·H2O}n (where 1,4-bpyvna and 1,3,5-H3BTC represent 1,4-bis(2-(pyridin-4-yl)vinyl)naphthalene and 1,3,5-benzene-tricarboxylic acid, respectively) as a Hg2+ sensor [104]. Due to the interactive effect of 1,4-bpyvna with Hg2+, the {[Zn2(1,4-bpyvna)(1,3,5-BTC)(OH)]·H2O}n in DMF displayed fluorescence quenching at 444 and 472 nm as seen in Figure 5.
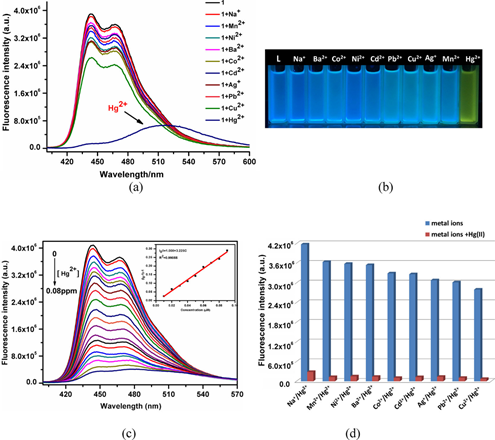
Figure 5. (a) Emission spectra of {[Zn2(1,4-bpyvna)(1,3,5-BTC)(OH)]·H2O}n in DMF in the absence/presence of Mn+ ions. (b) The colours of the suspensions of {[Zn2(1,4-bpyvna)(1,3,5-BTC)(OH)]·H2O}n with different metal ions under UV light. (c) Emission spectra of {[Zn2(1,4-bpyvna)(1,3,5-BTC)(OH)]·H2O}n in the presence of increasing Hg2+ concentrations (0–0.08 ppm) in DMF. Inset: linear relation between the quenching efficiency and the concentration of Hg2+ in the range of 0–0.018 ppm. (d) Fluorescence intensities of {[Zn2(1,4-bpyvna)(1,3,5-BTC)(OH)]·H2O}n immersed in the DMF solution of metal ion (blue colour) or mixed Hg2+ and metal ions (red colour) under an excitation of 389 nm (Reproduced with the permission from Ref [104]).
A linear response of the polymer was found from 0 to 0.018 ppm with a LOD of 0.057 ppm. A fluorescent colour change from blue to yellow was also observed. However, further interrogations for the BET surface area and real time applications are still required. Subsequently, Zhang and co-workers presented Hg2+ sensing ability of hydrothermally synthesized fluorescent coordination polymer, namely [Zn(H3TTA)(H2O)2]·2H2O (where H3TTA represents [2,2′:6′,2″-terpyridine]-4,4′,4″-tricarboxylic acid)), in aqueous solution [105]. Emission band at 500 nm was quenched in the presence of Hg2+ with a KSV value of 4695 M−1. This work is incomplete due to lack of information in the BET surface area, LODs, and practicality. Utilization of Zn- and Cd-based luminescent coordination polymers towards the quantitation of Hg2+ and Cr2O72- has been reported by many research groups [106,107,108]. Since Cr2O72- is a well-known source of Cr6+ ions, discrimination between them are not available in those reports. Therefore, those works cannot be considered as exceptional Hg2+ sensor studies. Similarly, Lin et al. demonstrated Pb2+ and Hg2+ sensing ability of Eu3+ containing coordination polymer, namely {[Ln2(PBA)3(H2O)3]·DMF·3H2O}n, in DMF and aqueous media, where PBA, DMF, and H2O represent deprotonated 5-(4-pyridin-3-yl-benzoylamino)-isophthalic acid, Dimethylformamide, and water molecules, respectively [109]. Wherein, the polymer can be used to detect Hg2+ in samples free of Pb2+. Thus, these MOFs can be accounted as Hg2+ sensors. However, they are non-selective.
In addition to metal coordination polymer-based Hg2+ sensor, Rachuri and co-workers reported a luminescent coordination polymer, namely [Zn(μ2-1H-ade)(μ2-SO4)] (by solvothermal reaction of adinine (HAde) and Zn(SO4)·7H2O), as discussed in the following [110]. In the report, fluorescence intensity of the [Zn(μ2-1H-ade)(μ2-SO4)] at 395 nm (in water) was linearly quenched between 0–1 mM of Hg2+ with a LOD of 70 nM and a KSV value of 7.7 × 103 M−1. Moreover, this polymer also has an additional advantage that it showed selective sensing of the 2,4,6-trinitrophenol (TNP) in aqueous medium. The underlying mechanism of detection of Hg2+ was attributed to the interaction with basic sites (N atoms) of the adenine and TNP through the resonance energy transfer (RET). It should be noted that this work also described the Hg2+ detection in paper strips, therefore, it can be extended for effective Hg2+ removal in real samples with directed BET surface area analysis. Thereafter, Zhu et al. demonstrated Hg2+ sensing utility of two luminescent coordination polymers, namely [Cd(L)(NTA)]n and [Ni(L)(NPTA)⋅H2O]n (obtained by solvothermal method, where L, H2NTA, and H2NPTA represent 1,6-bis(benzimidazol-1-yl)hexane, 2-nitroterephthalic acid, and 3-nitrophthalic acid, respectively) [111]. Emission peaks at 292 nm and 295 nm of the polymers [Cd(L)(NTA)]n and [Ni(L)(NPTA)⋅H2O]n (in water), respectively, were linearly quenched by Hg2+ in a concentration range between 1–200 µM with corresponding LODs of 3.05 μM and 2.29 μM and KSv values of 3565 M–1 for 1 and 7432 M–1. In addition, both polymers displayed selectivity only to acetylacetone among all solvents. However, this work requires information on the BET surface area, competing studies, mechanistic investigations, and practicality.
6. MOFs Holding Composites for Optical Recognition of Hg2+
To enhance sensing ability of MOFs to Hg2+, researchers also proposed to synthesize MOFs comprising composites as detailed in the following. A metal−polydopamine framework (MPDA—a dopamine loaded Co-based MOF developed by sonochemical reaction of Co(NO3)2 and 2-methylimidazole to afford ZIF-67 primarily) was reported as a fluorescent quencher towards the detection of Hg2+ and Ag+ via exonuclease III signal amplification activity with pico-molar level LODs (1.3 pM and 34 pM, respectively) [112]. Upon addition of Hg2+ to MPDA-T-rich ssDNA (T-rich ssDNA represents thymine rich single stranded Deoxyribonucleic acid) system, ‘turn-on’ PL emission enhancement at 520 nm was observed with a linear response from 0 to 2 nM and a LOD of 1.3 pM. The quenched luminescence of MPDA conjugated with T-rich ssDNA was recovered through T-Hg2+-T complexation during addition of Hg2+. This work was also well demonstrated in tap and river water applications. To this way, Huang and co-workers reported Cu-based MOFs as a hybrid sensory system with C-rich or T-rich DNA probes to detect the Ag+, Hg2+, and thiol comprising species at nanomolar-level via T-Hg2+-T complexation [113,114]. Wherein, Huang et al. developed a MOF, namely [Cu4(Dcbb)4(Dps)2(H2O)2]n (by reacting H2DcbbBr = 1-(3,5-dicarboxybenzyl)-4,4’-bipyridinium bromide and Dps (4,4’-dipyridyl sulfide) with Cu(NO3)2·3H2O), to detect Ag+, Hg2+, and biothiols with nM LODs [113]. Similarly, the MOF, namely [Cu(Cdcbp)(H2O)2·2H2O]n(synthesized by reacting H3CdcbpBr (3-carboxyl-(3,5-dicarboxybenzyl)-pyridinium bromide) with CuSO4·5H2O) was engaged in detection of Hg2+ and biothiols with nM LODs [114]. In both cases, the MOF tended to form a hybrid system initially with fluorescent dye loaded thymine rich (T-rich) DNA (labelled as P-DNA) which led to fluorescent quenching. It was then recovered upon addition of metal ions, Hg2+ in particular,viaT-Hg2+-T complexation. The above hybrid MOFs-DNA system-based detection of Hg2+ and biothiols and the corresponding mechanism are illustrated in Figure 6. Following the same strategy, Zr- and Ce-based MOFs (UIO-66-NH2 and Ce/TBC) were also demonstrated to discriminate Hg2+ with nanomolar LODs [115,116]. Wherein the Ce/TBC (also noted as mixed-valence state cerium-based metal-organic framework (MVC-MOF) combined with thymine-rich ssDNA was engaged in colorimetric peroxidase like sensors to detect Hg2+ using oxidase substrate 3,3′,5,5′-tetramethylbenzidine [116]. Results of Hg2+ detection showed a linear response in a concentration range of 0.05 to 6 μM with a LOD of 10.5 nM and were further supported by environmental water analysis. In fact, many MOF-DNA hybrid systems were reported for detection of metal ions, aminoacids, and nucleic acids [117,118], which make the strategy as precise one.
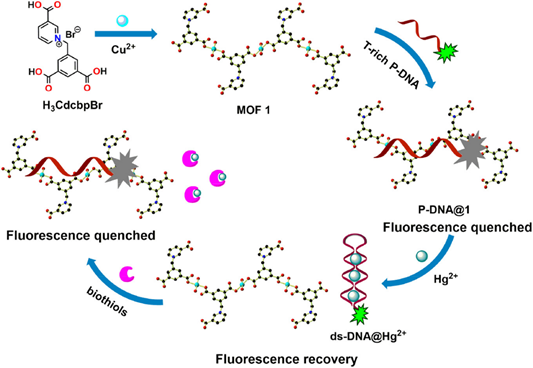
Figure 6. The proposed Hg2+ and biothiols detection mechanism based on the P-DNA@1 hybrid (Reproduced with the permission from Ref [114]).
Recently, gold nanoclusters composited MOFs, namely AuNCs/MIL-68(In)-NH2/Cys and AuNCs@UIO-66, were demonstrated in discrimination of Hg2+ [119,120]. To develop the AuNCs/MIL-68(In)-NH2/Cys, MIL-68(In)-NH2 was first solvothermally synthesized by reacting In(NO3)3·xH2O with 2-Aminoterephthalic acid (H2ATA) followed by evenly dispersing the AuNCs on its surface. The AuNCs exhibited emission bands at 438 nm and 668 nm (λex = 370 nm). By adding cysteine into above mixture, the AuNCs/MIL-68(In)-NH2/Cys was produced with enhanced emission [119]. Upon addition of Hg2+ to the AuNCs/MIL-68(In)-NH2/Cys at pH 7.4 (phosphate buffer), emission at 638 nm was quenched without affecting the peak at 438 nm. Due to the binding affinity of Hg2+ with thiol (-SH) of cysteine, PL quenching exhibited two linear responses (with a concentration range from 20 pM to 0.2 μM and from 0.2 μM to 60 μM) with a LOD of 6.7 pM, which was further supported by real water analysis and microfluidic paper device. Subsequently, the UIO-66 was obtained by solvothermally reacting zirconium chloride with 1,4-benzenedicarboxylic acid (H2BDC). The synthesized UIO-66 was then conjugated with AuNCs to afford AuNCs@UIO-66 with 11% quantum yield [120]. PL emission of the AuNCs@UIO-66 at pH 7.2 was observed at 650 nm and was quenched linearly by Hg2+ with concentrations from 800 nM to 10 μM and a LOD of 77 pM. The emission quenching was attributed to the Au-Hg amalgamation via interactive amino groups of bovine serum albumin (BSA) present over AuNCs surface. The applicability of the sensory study was also successfully demonstrated in tap and river water which can be accounted as a good candidate for Hg2+ discrimination.
To this track, Marieeswaran and co-workers presented Hg2+ sensing ability of the MOF containing composite consisted of magnetic nanoscale metal–organic framework (MNMOF) conjugated with fluorescein amidite (FAM)-labeled ssDNA [121]. The MNMOF was developed by one-pot reaction between Fe3O4 nano-spheres (synthesized hydrothermally), FeCl3.6H2O, and 2-aminoterephthalic acid. Emission intensity at 495 nm (in Tris-HCl buffer) was quenched up to 62% with adsorption of FAM-labeled ssDNA on the MNMOF. Addition of Hg2+ further lowered the emission quenching at 495 nm down to 52% via T-Hg2+-T complexation. The linear regression of Hg2+ detection was between 2–20 nM with a LOD of 8 nM. This work was also demonstrated in environmental water samples. However, the BET surface area information and competing studies still need to be updated. In addition to the MOF-DNA hybrid system, Hu and co-workers revealed the utilization of the {[Cu(Dcbb)(Bpe)]·Cl}n (where H2DcbbBr and Bpe represent 1-(3,5-dicarboxybenzyl)-4,4′-bipyridinium bromide and trans-1,2-bis(4-pyridyl)ethylene, respectively) towards sequential quantitation of Hg2+ and I- via the T−Hg2+−T motif and “off-on-off” fluorescence response [122]. Detection of Hg2+ and I- was verified by circular dichroism (CD) and the underlying mechanism was clarified by fluorescence anisotropy, binding constant, and simulation studies. LODs for Hg2+ and I- sensors were estimated as 3.2 and 3.3 nM, respectively. Though the sensor showed high selectivity, it can be considered a supplementary work due to the similar T−Hg2+−T motif approach.
The AuNP@MOF composite nanoparticles were used in colorimetric Hg2+ assay via gold amalgam-triggered reductase mimetic activity in aqueous medium [123]. The AuNP@MOF composite was developed by immobilizing Au NPs over the porous surface of iron-5,10,15,20-tetrakis (4-carboxyl)-21H,23H-porphyrin-based MOF- (Fe-TCPP-MOF) and was utilized in colorimetric sensing of Hg2+. Wherein, Hg2+ triggered the Au-mediated catalytic reduction of methylene blue and turned the blue colour to colourless, which was accompanied with quenching in absorbance peak of methylene blue at 665 nm as shown in Figure 7. Moreover, the absorbance at 665 nm was linearly quenched within 2s by Hg2+ with concentrations from 200–400 pM and a LOD of 103 pM. Apart from lack of the BET surface area information, the competing studies, tap and river water investigations attested the utility of the AuNP@MOF in Hg2+ detection.
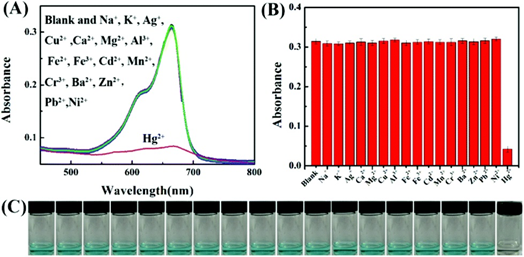
Figure 7. Selectivity of the sensing method by AuNP@MOF to other metal ions. Concentrations of Hg2+ and other metal ions (Na+, K+, Ag+, Ca2+, Mg2+, Cu2+, Al3+, Fe3+, Fe2+, Cd2+, Mn2+, Cr3+, Ba2+, Zn2+, Pb2+ and Ni2+) were 1 nM and 10 nM, respectively. (A) The UV spectra of the sensing system in response to various metal ions; (B) The UV absorbance of the sensing system at 665 nm towards various metal ions; (C) photographs of the sensing system responding to various metal ions (Reproduced with the permission from Ref [123]).
In this light, Zhang et al. deposited Au nanoparticles on titanium-based MOF (NH2-MIL-125(Ti)) by solvothermal reaction between 2-aminoterephthalate and tetrabutyl titanate) to afford Au@NH2-MIL-125(Ti) for colorimetric detection of H2O2, cysteine, and Hg2+ via peroxidase-like activity using 3,3′,5,5′-tetramethylbenzidine (TMB-catalyst) [124]. The Au@NH2-MIL-125(Ti) and TMB showed a blue colour and a “turn-on” absorbance response at 652 nm in the presence of H2O2 via peroxidase like mimic. Upon addition of cysteine to above sensory system, absorbance at 652 nm was quenched accompanied with disappearance of the blue colour. However, the blue colour and absorbance peak were recovered by adding Hg2+. The Hg2+ detection showed a linear response in concentrations from 1 to 5 µM with a LOD of 100 nM and was authenticated by real sample analysis. A masking tactic with EDTA was proposed in this report to avoid the interference effect from other metal ions, however, this work required more complicated detection procedures.
A “CDs/AuNCs@ZIF-8” composite consisted of zeolitic imidazolate framework-8 (ZIF-8) encapsulated with carbon dots (CDs) and gold nanoclusters was produced and utilized in detection of Hg2+ [125]. The CDs/AuNCs@ZIF-8 in aqueous medium displayed emission bands at 440 and 640 nm (λex = 360 nm) due to the encapsulated CDs and AuNCs. In the presence of Hg2+, PL peak at 640 nm was quenched but emission band at 440 nm was not affected. Therefore, it was noted as a ratiometric sensor. Due to the Au-Hg amalgamation, the probe showed linear ratiometric response (I640/I440) between 3–30 nM with a LOD of 1 nM accompanied with visualization of red blue emission colour changes under UV-irradiation. This work was also demonstrated in tap and river water samples. Following the ratiometric sensing approach, Yang et al. constructed a ratiometric sensor MOF/CdTe QDs via physically mixing CdTe QDs (λem = 605 nm) with MOF (Fe-MIL-88NH2, λem = 425 nm) towards Hg2+and Cu2+ discrimination [126]. During detection of metal analytes, ratiometric responses at I425/I605 due to the strong binding interaction between CdTe QDs and metal ions were evaluated by monitoring the colour changes (from pink to blue). Although this work was well supported by paper-based analysis, lake water, fruit juice, and red wine samples, but it lacks information in competing studies.
7. MOFs in Electrochemical Recognition of Hg2+
Similar to many inorganic electrochemical sensors [127], MOFs were also engaged in electrochemical-based detection of Hg2+ as described subsequently. For example, Zhang et al. demonstrated electrochemical sensing of Hg2+ in the presence of glucose by using the Cu2+ anchored MOFs as enzyme free catalyst [128]. MOFs were firstly synthesized By solvothermally reacting amino terephthalic acid (NH2-BDC) with Cu(NO3)2. The as-synthesized MOFs were then combined with Au NPs and sDNA to obtain the sDNA/MOF-Au probe. The cDNA/GO@Au/GCE electrode was immersed in the mixed solution of sDNA/MOF-Au and Hg2+ at certain concentration for Hg2+ sensing. The sDNA/MOF-Au probe detected Hg2+ via T-Hg2+-T coordination and induced a oxidase-like catalytic response. This work showed a linear response to Hg2+ in concentrations from 0.10 aM to 100 nM (aM = attomole = 10−18 M) with a LOD of 0.001 aM. Moreover, this work was also demonstrated in dairy product samples, which was impressive. However, careful optimization is required to attain the reproducible results. Recently, the ZIF-8 was synthesized via hydrothermal reaction of Zn(NO3)2⋅4H2O and 2-methylimidazole and employed in electrochemical discrimination of Hg2+ by coupling with Ag and Au nanoparticles [129]. This work also involved the electrochemical aptasensor based on the Au electrode (AE). Signals were obtained from the “APT/Ag@Au/ZIF-8/AE” aptasensor by means of differential pulse voltammetry (DPV) and electro-chemical impedance spectroscopy (EIS). Due to the T-Hg-T complexation from T rich aptamer, Hg2+ detection became feasible. Linear responses were observed from detection of Hg2+ in concentrations from 1.0 × 10−16 to 1.0 × 10−12 M and from 5.0 × 10−15 to 1.0 × 10−12 M with LODs of 1.8 ± 0.04 × 10−17 M and 1.3 ± 0.01 ×10−16 M by DPV and EIS, respectively. This work was well supported by real water samples, thereby can be considered a report in T-Hg2+-T motif featured electrochemical sensors.
Similar results were reported from many MOFs-based electrochemical sensors with fM LODs [130,131,132]. Wherein, the Cu-based MOFs (Cu-MOFs), thioether side groups attached Zr-based MOFs (S-Zr-MOFs), and 2D-Co-based MOFs (PPF-3 nanosheets) were engaged in the fabrication of Cu-MOFs/Au, S-Zr-MOF/SPE (SPE represents screen printed electrode), and anchor−Au NPs@PPF-3 attached DNB/depAu/GCE (Au NPs, anchor, anchor, DNB, and depAu/GCE represent gold nanoparticles, dibenzocyclooctyne (DBCO), 3D DNA “nanosafe-box”, and gold nanoparticle-coated glassy carbon electrode, represtively) electrodes towards the specific detection of Hg2+ ions. The GCE/AuNPs/DNA2 sensor was incubated in a mixture of Cu-MOFs/DNA1 probes (T-rich DNA used in study) and Hg2+. It demonstrated a linear DPV response from 10 fM to 100 nM with a LOD of 4.8 fM and also real time applications [130]. Similarly, the S-Zr-MOF/SPE showed a linear differential pulse anodic stripping voltammetry (DPASV) response for Hg2+ between 0.03 nM to 3 µM with a LOD of 7.3 fM via multiple Hg-S interaction (by thio-ether side chains) and T-Hg2+-T formation [131]. By dropping the anchor−AuNPs@PPF-3 [the PPF-3 was synthesized by solvothermally reacting Co(NO3)2·6H2O, 5,10,15,20-tetrakis(4-carboxyl-phenyl)-porphyrin (TCPP), and 4'-bipyridine (BPY)] over the surface of DNB/depAu/GCE, the proposed electrode fabrication was completed. The electrode was subjected to Hg2+ detection to reveal a linear DPV response between 0.1 pM to 10 nM with a LOD of 33 fM [132]. Next, the Cu-MOF-mediated Hg2+ detection by means of differential pulse voltammetry (DPV) and cyclic voltammetry (CV) tactics in 0.1 M phosphate buffer (PB) at pH 9 was reported by Singh and co-workers [133]. In the report, CuNO3·3H2O and 2-aminoterephthalic acid were solvothermally reacted to afford porous Cu-MOF nanoparticles, which could absorb large amount of Hg2+. Through coupling with the GCE, the Cu-MOF detected Hg2+ linearly in concentrations from 0.1 to 50 nM with a LOD of 0.0633 nM. Reliability of this work was well demonstrated by the real tuna-fish and tap water samples investigations.
Thereafter Kokkinos and co-workers proposed utilization of the Ca-MOF modified working electrodes towards 3D-printed lab-in-a-syringe voltammetric cell mediated electrochemical detection of Hg2+ [134]. The Ca-MOF, namely [Ca(H4L)(DMA)2]·2DMA, where H6L and DMA represent N,N’-bis(2,4-dicarboxyphenyl)-oxalamide and N,N-dimethylacetamide), showed exceptional selectivity to Hg2+. Moreover, the voltammetric lab-in-a-syringe device consisted of a vessel assimilating two thermoplastic conductive electrodes (act as counter and pseudo-reference electrodes) was fabricated by a single-step process. A small detachable 3D-printed syringe loaded with a graphite paste/Ca-MOF mixture was used as a working electrode. The Ca-MOF participated in the ion exchange to form “Hg@CaMOF”. The device showed a linear response to Hg2+ with concentrations between 2–40 μg L−1 and a LOD of 0.6 μg L−1. Practicality of this work was also demonstrated in the fish oil and bottle water samples.
In contrast to direct electrochemical sensors, Zhang et al. proposed the MOF-involved light driven electrochemical (photoelectrochemical; PEC) sensor for Hg2+discrimination as described next [135]. Firstly, Co(NO3)2·6H2O and 2-methylimidazole were solvothermally reacted to afford the ZIF-67. The ZIF-67 was then reacted with Cd(NO3)2·4H2O and thioacetamide (TAA) to yield the CoSx@CdS nanocomposites (CdS nanoparticles were grown over the surface of cobalt sulfide (CoSx) by using ZIF-67 polyhedrons as the sacrificial templates and cobalt precursors). The synthesized composite was drop casted on the ITO electrodes to engage in the photoelectrochemical sensing of Hg2+ as illustrated in Figure 8.
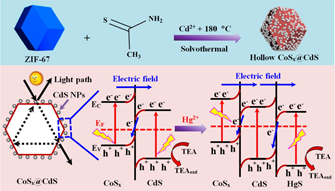
Figure 8. Synthetic process of hollow CoSx polyhedrons and CoSx@CdS composites and the mechanism of photocurrent responses of CoSx@CdS composites, showing the band structures of CoSx@CdS/HgS composites and charge separation under the visible-light illumination (Reproduced with the permission from Ref [135]).
In the presence of Hg2+ions in PBS (pH 7.4) containing 0.15 M triethanolamine (TEA) (see Figure 8), carrier transport and photocurrent of the device were improved and enhanced under illumination due to the combined CoSx and CdS components. In fact, the ion-exchange reaction took place to trigger in-situ generation of CoSx@CdS/HgS (new Z-scheme heterojunction photocatalyst) during the detection process. This sensor showed a linear response to Hg2+ with concentrations between 0.010–1000 nM and a LOD of 2 pM. It was also validated by tap and lake water analysis.
8. MOFs in Removal of Hg2+
Other than optical and electrochemical recognition of metal analytes, MOFs were also engaged in the selective removal heavy metal ions [136]. In this section, removal/extraction of Hg2+ in particular was described in detail. Many Zr-based MOFs displayed great adsorption capacity to Hg2+ due to their porous nature. For example, Kahkha and co-workers described the consumption of mesoporous porphyrinic containing Zr-MOF-PCN-222/MOF-545 for effective pipette-tip solid-phase extraction of Hg2+ [137]. The MOF was solvothermally synthesized by reacting meso-tetrakis(4-carboxyphenyl)porphyrin (H2TCPP) and ZrOCl2·8H2O. The as-synthesized MOF was then inserted into a Bio Plas pipette-tip attached to a 2000-μL micro pipette for Hg2+ adsorption. It was shown that 2 mg of MOF- sorbent was enough to accomplish extraction and desorption of Hg2+ up to 15 cycles at pH 5 by using 10% HCl as eluting solvent (fixed at 15 µL volume). A LOD of this method was estimated as 20 ng L−1 with an adsorption capacity (contrast to other ions), enrichment factor, and total extraction time of 35.5 mg g−1, 120-fold, and 7 min, respectively. This work was demonstrated for Hg2+ determination in the fish samples, however, information regarding the BET surface area and Langmuir/Freundlich isotherms requires further interrogations. Hasankola et al. engaged the MOF-PCN-221 (synthesized by solvothermal reaction), which comprised of 5,10,15,20-tetrakis(4-carboxyphenyl) porphyrin (H2TCPP) organic linker and ‘Zr’ metal node, towards selective removal of Hg2+ [138]. Wherein the time required for Hg2+adsorption was 30 min at an optimal pH of 7.1 and an adsorption capacity of PCN-221 was established as 233 mg g−1. Be noted that the adsorption process of Hg2+ by PCN-221 can be properly interpreted with the pseudo-second-order kinetic model (with R2 = 0.99) and followed the Langmuir model adsorption isotherms for Hg2+. Investigations on the Hg2+ adsorption mechanism indicated that Hg2+ could not be replace with Zr ions. For the desorption process, 0.01 M HNO3 was used. This work requires more investigations for the BET surface area, practicality, and structural features.
Li et al. described the utilization of porous and highly defective Zr-based MOF-UIO-66-Zr6(OH)4O4(BDC)6 (where BDC represents benzene-1,4-dicarboxylate), namely UIO-66-50Benz (Benz represents benzene-1,4-dicarboxylate), for Hg2+removal [139]. By means of topotactic transformations of MOFs and ligand extraction process, ZrOx (obtained by immersion of UIO-66-50Benz in 10 mol L−1 NaOH), ZrOxyPhos (obtained by immersion of UIO-66-50Benz in 210 mM Na3PO4), and ZrSulf (obtained by immersion of UIO-66-50Benz in 250 mg of Na2S solution in 10 mL) were developed and engaged in Hg2+ removal as seen in Figure 9.
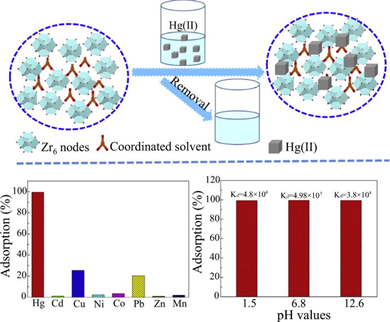
Figure 9. Schematic of the utilization of highly defective Zr-based porous MOFs- ZrOx, ZrOxyPhos, and ZrSulf towards Hg2+ removal and excellent Hg2+ removal performance by ZrSulf compared to other metal ions and at different pH ranges; coordinated solvents NaOH, Na3PO4, and Na2S were used to affordZrOx, ZrOxyPhos, and ZrSulf, respectively. (Reproduced with the permission from Ref [139]).
The BET surface area of ZrOx, ZrOxyPhos, and ZrSulf were estimated as 430, 290, and 560 m2 g−1, correspondingly, which also confirmed the high effectiveness of ZrSulf. Among these materials, ZrSulf possessed the fastest adsorption kinetics (1.1 × 10−2 g (mg min)−1 and the highest adsorption capacity of 824 mg g−1. The distribution coefficient (Kd) of ZrSulf to Hg2+ was estimated as 4.98 × 105 mL.g−1 at pH 6.8 Moreover, it was reusable for more than five cycles after washing with HCl and thio-urea. The high selectivity of Hg2+ was attributed to the covalent bond formation with sulfur-based functionality. From kinetic studies, it was established that the adsorption followed the pseudo-second order model and, at the same time, controlled by the film diffusion and pore diffusion. This material can be considered as a good candidate for Hg2+ removal in terms of its adsorption capacity and practicality.
Subsequently, Leus and co-workers solvothermally synthesized the thiolated Zr-based MOF-UIO-66-(SH)2 (by reacting ZrOCl2.8H2O and 2,5-dimercaptoterephthalic acid-(H2BDC-2,5SH)) and applied it for selective removal of Hgspecies [140]. The Langmuir surface area of UIO-66-(SH)2 was estimated as 499 m2 g−1. The UIO-66-(SH)2 showed a maximum Hg2+ adsorption capacity of 236.4 mg g−1 between pH 3.0–5.0. Due to the presence of -SH group, adsorption of Hg2+ showed the best fit with Langmuir isotherm and followed the pseudo-second order kinetics. Moreover, adsorption and desorption of Hg2+ can be extended up to three cycles by using 1 M HCl and 0.66 M thiourea. This work was also applied in waste water-based Hg2+ removal. The use of thiolated UIO-66-SH (an archetypal thiolated Zr-based MOF- Zr6(OH)4O4(BDC)6, where BDC represents benzene-1,4-dicarboxylate) towards Hg species removal applications was also demonstrated by Li and co-workers [141]. However, the presence of Zn2+ and Pb2+ may reduce the adsorption of Hg2+ by UIO-66-SH.
Next, Fu et al. employed the post-functionalized UIO-66-NH2 (Zr-based MOF) with 2,5-Dimercapto-1,3,4-thiadiazole to produce the UIO-66-DMTD for effective removal of Hg2+ in water [142]. Due to the Hg2+ adsorption over the MOF surface, the calculated BET surface area of UIO-66-DMTD-Hg decreased from 651 m2 g−1 to 42 m2 g−1, thereby confirming the adsorbing ability of the proposed MOFs. The maximum adsorption of Hg2+ was 670.5 mg g−1 at pH 3. The adsorption kinetic followed the pseudo-second-order and was linearly fitted with Langmuir isotherm. Moreover, selectivity to Hg2+ by the UIO-66-DMTD and its analogous (UIO-66-NH2 and UIO-66-SO3H) was higher than that of other species as seen in Figure 10.
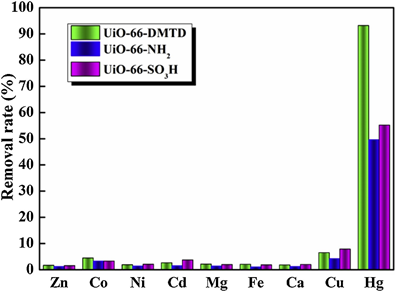
Figure 10. Selective removal of Hg2+ by UIO-66-NH2, UIO-66-SO3H and UIO-66-DMTD (Reproduced with the permission from Ref [142]).
At a fixed Hg2+ removal time of 120 min, recyclable usage of the UIO-66-DMTD was found to be effective up to 10 times. The Hg2+ removal is highly fascinated and can be effective in the lab wastewater-based extraction due to the high affinity of thiol (-SH) group to Hg2+.
Similar to the MOF-UIO-66-(SH)2 [140], Ding and co-workers proposed the Zr-based MOF-Zr-DMBD (synthesized by reacting 2,5-Dimercapto-1,4-benzenedicarboxylic acid (H2DMBD) and ZrCl4) for Hg2+ removal [143]. However, due to a high degree of similarity to the UIO-66-(SH)2-based research, this work will not be further discussed. Regarding the effectiveness of thiol functionalized or thiol comprising MOFs towards removal of Hg2+ species, Li et al. described the utilization of dense thiol arrays containing Zr-based MOF, namely ZrOMTP (via reacting 4,4′,4″,4‴-(pyrene-1,3,6,8-tetrayl)tetrakis(2,6-dimercaptobenzoic acid)(H4OMTP) with ZrCl4), in Ref. [144]. The BET surface area of ZrOMTP to N2 gas was estimated as 1290 m2 g−1 and the distribution coefficient (Kd) for Hg2+ was calculated as 1.60 × 108 mL g−1, which is far better than that of other thiol containing MOFs. This could be attributed to the dense thiol arrays present in ZrOMTP framework. Adsorption of Hg2+ followed the first order kinetic model and was best fitted with Langmuir isotherms. Moreover, this MOFs lowered Hg-based contaminants from ppm to below the allowed drinkable limit of 2 ppb.
Highly dense alkyl thiol comprising MOF-Zr-MSA was developed by hydrothermally reacting ZrCl4 and mercaptosuccinic acid (HOOC-CHSH-CH2-COOH, MSA) in aqueous phase and was engaged in Hg2+removal [145]. The Zr-MSA showed an adsorption efficiency of 99.99% to Hg2+ in a pH range of 0–7 pH within 5 min and was reusable (with 6 M HCl) for up to five cycles. Moreover, the Zr-MSA showed a maximum adsorption capacity of 734 mg g−1 and a Kd value of 1.82 × 108 mL.g−1, which was best correlated with Langmuir isotherm. Due to the higher affinity of -SH to Hg2+, this work reduced the Hg content from 10 000 ppb to 0.11 ppb, which was far below the drinking water limit. In addition to thiol containing MOFs, the Zr-MOFs (Zr-MOFs-SH(O)) was synthesized by one-pot reaction of ZrCl4, meso-tetra(4-carboxyphenyl)porphine (H2TCPP), and modulators—mercaptoacetic acid (MAA) or alpha lipoic acid (ALA)—and was employed in Hg2+ adsorption [146]. For comparison, the Zr-MOFs-SH(P) was synthesized via post-synthetic modification of the Zr-MOFs-SH(O) and was engaged in adsorption studies. Due to the higher -SH content in the Zr-MOFs-SH(O), it showed a higher adsorption capacity (for Hg2+) of 843.6 mg g−1 than that of the Zr-MOFs-SH(P) (138.5 mg g−1). Hg2+ adsorption of the Zr-MOFs-SH(O) followed the pseudo-second-order kinetic model and was best fitted with Langmuir isotherm. In addition, this study showed good selectivity, recyclability, and chemical stability. By functionalizing the NH2-UIO-66 with L-cysteine, the Cys-UIO-66 was obtained and was used for Hg2+ removal from solution [147]. The Cys-UIO-66 showed a maximum adsorption capacity of 350.14 mg g−1 (after 180 min) at pH 5.0 of Hg2+ adsorption which followed the pseudo-second-order model and was fitted with Langmuir isotherm. Due to the -SH (from cysteine) affinity to Hg2+, reusability of Hg2+ adsorption/desorption was up to five cycles (with 0.1 M HNO3 and 1% thiourea solution). In terms of the capacity and time consumption, this study could need more improvements. By following the similar approach, Liu and co-workers presented the cysteamine functionalized MOFs-MIL-101-SH (Cr) and UIO-66-SH (Zr) for Hg2+ removal [148]. The MOFs showed adsorption capacities of 10 and 250 mg g−1 at pH of 5, respectively, with certain reusability.
By utilizing four different types of organic ligands with bulky sulphur side chains, four Zr-based MOFs, namely Zr-L1, Zr-L2, Zr-L3, and Zr-L4 (Zr(IV)-carboxylate frameworks; where L1-L4 represents the deprotonated four thioether-equipped carboxylic acid linker molecules), were constructed for selective removal of Hg2+ ions [149]. The Kds values of Zr-L1, Zr-L2, Zr-L3, and Zr-L4 were estimated as 1.95 × 103 mL.g−1, 1.47 × 104 mL.g−1, 4.47 × 103 mL.g−1, and 2.40 × 104 mL.g−1, respectively. Moreover, Hg adsorption of Zr-L1, Zr-L2, Zr-L3, and Zr-L4 followed the Langmuir isotherm with capacities of 193 mg g−1, 275 mg g−1, 245 mg g−1, and 322 mg g−1 at pH 6.8, correspondingly. However, this work needs further investigations on interference studies and real time applications. The Zr-based MOF-DUT-67 (Zr) synthesized by solvothermal reaction of zirconium chloride with 2, 5-thiophene-dicarboxylic acid showed removal efficiencies for Hg2+ and CH3Hg+ from 69% to 90% and from 30% to 77%, respectively [150]. At pH 6, the DUT-67 (Zr) showed a great efficiency to Hg2+ and CH3Hg+ with adsorption capacities of 0.0451 mg g−1 and 0.0374 mg g−1, respectively. The adsorption followed the pseudo-second-order kinetic model. This work was also demonstrated in river and lake water samples, but mechanism and interference studies require further investigations. The ZrO2-based (MOF)-808 synthesized by a sol-gel method was grafted with amidoxime (AO) via wet-chemistry process to afford MOF-808/AO, which was used in Hg2+ removal and displayed high efficiencies [151]. In particular, the MOF-808/AO showed a higher adsorption efficiency in all pHs. The BET surface area of MOF-808 and MOF-808/AO were established as 2152 and 1899 m2 g−1, respectively. Moreover, adsorption capacities of MOF-808 and MOF-808/AO towards Hg2+ were estimated as 383.8 mg g−1 and 343.6 mg g−1 (at 70 min), correspondingly. The Hg2+ adsorption in both MOFs followed the pseudo-second-order kinetic model and was fitted with Langmuir isotherms. This work requires more efforts to obtain additional information on the mechanism, interference effect, and real time analysis.
The Zr-based MOFs and Zn-metal nodes comprising MOFs were also engaged in Hg2+ removal as detailed next. The Zn2(DHBDC)(DMF)2·(H2O)2, namely MOF-74-Zn, was synthesized by solvothermally reacting ZnNO3 and 2,5-dihydroxy-1,4-benzenedicarboxylic acid (DHBDC) and was applied in Hg2+ removal [152]. The MOF-74-Zn showed a maximum adsorption capacity of 63 mg g−1 (for Hg2+ at pH 6 in 90 min). The Hg2+ adsorption followed the pseudo-second-order kinetic model but was best fitted with the Langmuir isotherm rather than the Freundlich isotherm. The -OH group was directly involved in adsorption of Hg2+. However, this work showed a minimum adsorption capacity and lacked information on the interference effect. Wang and co-workers presented the Zn-based MOF, namely NTOU-4 (hydro(solvo)thermally synthesized by reacting ZnNO3 with 1H-1,2,4-triazole-3,5-diamine and 1,4-benzenedicarboxylate organic linkers) for Hg2+ removal applications [153]. The NTOU-4 showed an adsorption capacity of 163 mg g−1 at 30 min and was operable between pH 3–11. However, the underlying mechanism, kinetic model, and isotherm studies require further clarification. Next, Esrafili et al. described the utilization of dual functionalized Zn-based MOF, namely TMU-32S (synthesized by incorporation of different percentile of N1,N3-di(pyridine-4-yl) malonamide in TMU-32 (a Zn containing MOF with urea linkers)), towards Hg2+ adsorption and removal [154]. Due to the strong binding forces produced by urea and malonamide functional units, the TMU-32S showed a high adsorption capacity of 1428 mg g−1 (in just 17 min) and became more efficient at pH 4.4. The system followed the linear pseudo-second-order model and was linearly fitted with the Langmuir isotherm. Moreover, the material showed adsorption and desorption (with 0.2 M of EDTA) up to three cycles with 65% efficiency. This work requires more studies regarding the interference effect with several metal analytes.
Subsequently, the Cu-based MOFs were authorized as efficient adsorbents for Hg2+ removal as described next. Wu et al. developed the copper and 3,30,5,50-azobenzenetetracarboxylic acid containing porous MOF, namely JUC-62, for Hg2+removal in tea and mushroom samples [155]. The adsorption capacity of the JUC-62 to Hg2+ was established as 836.7 mg g−1 at pH 4.6 in 15 min in aqueous media. This work followed the pseudo-second-order model and was fitted with Langmuir adsorption isotherm. Moreover, it was reusable with EDTA up to four cycles. However, further interrogations are required on the interference studies. Mon and co-workers described utilization of a Cu-based MOF, namely {Cu4II[(S,S)-methox]2}.5H2O (where methox represents bis[(S)-methionine]oxalyl diamide), for HgCl2 removal studies [156]. This microporous MOF was decorated with thioalkyl chains, thereby was able to adsorb HgCl2 efficiently to afford the HgCl2S2 adduct. This MOF adsorbed 99.95% of HgCl2 within 15 min and reduced the Hg2+ concentration from 10 ppm to below 2 ppb in drinking water. However, this work lacked information on the reusability, kinetic studies, and real applications. Next, the polysulfides functionalized benzene-1,3,5-tricarboxylic acid and Cu containing Sx-MOF (where MOF represents Cu-BTC (by solvothermal tactic) and Sx2−, X = 3, 4, 6) were described for efficient adsorption of Hg2+ [157]. Among these materials, the S4-MOF displayed great selectivity to Hg2+ with a LOD of 0.13 μg L−1 and a linear response from 30–200 μg L−1 at pH 6 in 30 min. The S4-MOF showed different adsorbing capacities to different metal ions in the following orders: Hg(II) > > Pb(II) > Zn(II) > Ni(II) > Co(II). By means of Hg2+-S bonding, adsorption was efficient and applicable in sea, tap, and wastewater. However, information regarding kinetic studies is still missing. A copper metallacycle complex, namely Cu2(PDMA)2(DMF) (comprised of 3,3’-((1E,1’E)-(pyrimidine-4,6-diylbis(2-methylhydrazin-2-yl-1-ylidene)) bis (methanylylidene)dibenzoic acid (H2PDMA)), was demonstrated for Hg2+ removal [158]. Due to the multi ‘N’ binding sites, the MOF showed an adsorption efficiency of 61.4% for Hg2+ (among Hg2 +, Mn2 +, Cd2 +, Pb2 + ions) with an adsorption capacity of 300 mg g−1. Moreover, this MOF was reusable with EDTA. The Hg2+ adsorption followed the pseudo-second-order kinetic model. Xu and co-workers proposed utilization of the SH@Cu-MOF towards adsorption of Hg2+and Hg(0) species by grafting dithioglycol from the post-synthetic modified Cu-MOF (Cu with 5-aminoisophthalic) [159]. Though the material seems to be impressive compared to others reports, however, its adsorption capacity (173 mg g−1 in 6 h)was not up to standard. However, this work does point to a new direction for future development of the Cu-based MOFs.
Liang and co-workers described utilization of the sulfur-functionalized Co-based MOF, namely FJI-H12 (composed of NCS-, Co(II) and 2,4,6-tri(1-imidazolyl)-1,3,5-triazine (Timt)), for Hg2+ removal in water [160]. The FJI-H12 showed a Kd value of 1.85 × 106 mL.g−1 with an adsorption capacity of 439.8 mg g−1 at pH 7. The adsorption was efficient because of the Hg2+ to S (of SCN-) affinity and it could be applied for continuous removal purpose. Moreover, this work followed the pseudo-second-order kinetic model and was also reusable, thereby is attested a nice work. Jiang et al. designed a stable sulfur containing Co-based MOF {[Co3(μ3-OH)(DMTDC)3(INT)3]-[Co2(OH)(H2O)2](NO3)19-(H2O)7(DMA)11}n, namely NENU-401 (where DMTDC, INT, DMF, and DMA represent 3,4-dimethylthieno[2,3-b]thiophene-2,5-dicarboxylic acid, isonicotinate, N,N-dimethylformamide, and N,N-dimethylacetamide), via introducing an INT group in NENU-400-{[Co3(μ3-OH)(H2O)3(DMTDC)3](NO3)10-(H2O)6(DMF)6} and utilized it successfully in Hg2+removal [161]. Unlike the NENU-400, which collapsed easily during Hg2+ adsorption, the NENU-401 preserved its structural features, thereby was highly applicable for Hg2+ extraction. The Kd value of NENU-401 at 25 °C was estimated as 8.3 × 106 mL g−1 with an adsorption capacity of 596.57 mg g−1 in 10 min. The NENU-401 performed far better than many thiol containing MOFs. Moreover, Hg2+ extraction by the NENU-401 was recovered up to 90% of its original by thioglycol solution and was reusable for more than four cycles because of the effective coordination between Hg2+ and ‘-S’ atom. Note that the NENU-401-based Hg2+ extraction followed the pseudo-second-order kinetic model and was linearly fitted with Langmuir isotherm. This work demonstrated an impressive approach to improve the structural stability of MOFs. Moreover, it also displayed certain selectivity to Pb2+ (nearly 70%) but still required further optimization. Recently, Sun’s research group proposed employment of the sulfur-rich two-dimensional (2D) Co-based MOF nanosheets, namely 2D-NCS ({[Co(NCS)2(pyz)2]}n; where pyz represents pyrazine), for exceptional removal of HgCl2 [162]. The BET surface area of 2D-NCS to N2 gas at 77K was established as 365 m2 g−1 with a maximum adsorption capacity of 1698 mg g−1 in 15 min and a Kdvalue of 2.26 × 106 mL g−1. The MOF nanosheets reduced Hg2+ concentrations from 10 ppm to 1 ppb within 15 min and were also effective in environmental samples between pH 4–9. This work followed the pseudo-second-order model and was fitted with Langmuir isotherm. Due to the strong Hg–S interactions, extraction was efficient up to five cycles (by thioglycol solution) and could be further tuned towards development of 3D materials for future environmental remediation. Similar to the FJI-H12 [152], another Co-based MOF- [Co3(SCN)6(TPMA)4]n, namely FJI-H30 (synthesized by solvothermally refluxing TPMA (tris(pyridin-4-ylmethyl)amine) and Co(SCN)2), was engaged in Hg2+ adsorption [163]. Due to the exceptional interaction between SCN− groups to Hg2+, its maximum adsorption capacity reached 705 mg g−1 with negligible interference. The BET surface area of FJI-H30 to CO2 gas at 195K was determined as 221 m2 g−1. This material showed a Kdvalue of 1.84 × 105 mL g−1 and operated efficiently between pH 4–9 with regeneration (by KSCN solution) of > 90% up to three cycles. This work followed the pseudo-second-order model and was fitted with Langmuir isotherm. It can be applied in industrial waste water, thereby is considered a nice work.
Halder et al. engaged the thiocyanato ligand (SCN−) comprising Ni-based 3D MOF, namely [Ni(3-bpd)2(NCS)2]n (where 3bpd represents 1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene), for effective removal of Hg2+ in aqueous solution [164]. Because the uncoordinated S atom of SCN− was strongly bonded with Hg2+ and formed the mercuric thiocyanate adduct, therefore, a great adsorption capacity of 713 mg g−1 was achieved. Nevertheless, this work still requires further optimization for the interference effect, adsorption kinetics, and practicality. A post-synthetic modified tactic was proposed to develop the thiol (-SH) functionalized In-based MOF, namely SH-MIL-68(In) (primarily NH2-MIL-68(In) obtained by solvothermally reacting 2-amino-benzene-1, 4-dicarboxylic acid (NH2-H2BDC) with In (NO3)3 followed by post-synthetic modification), towards Hg2+ extraction [165]. The SH-MIL-68(In) showed a highest Hg2+ adsorption capacity of 450 mg g−1 and a large adsorption rate (rate constant k2 = 1.25 g mg−1 min−1). As seen in Figure 11, the adsorption process took place within 2 min at pH 4 due to the presence of free -SH group.
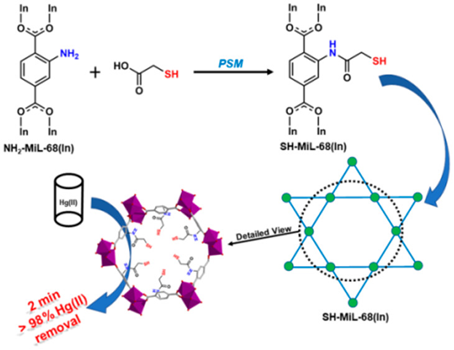
Figure 11. Schematic of post-synthetic modification of NH2-MIL-68(In) to afford SH-MIL-68(In) and its utilization in Hg2+ extraction (Reproduced with the permission from Ref [165]).
The material was reusable (in the presence of 0.01 M HCl, 0.1% thiourea) up to five cycles. This work followed the pseudo-second-order model and was linearly fitted with Langmuir isotherm. It is an impressive work considering its short process time and negligible interferences. However, real time applicability still needs to be demonstrated. By means of diffusion and solvothermal strategies, Li et al. developed three thioether-based MOFs, namely [(ZnCl2)3(L1)2·χ(solvent)]n-(1), [(Cu2I3O2)4(CH4N0.5)4(L1)4(DMA)4·3(H2O)·χ(solvent)]n-(2), and [(CuBr2)2(L2)2 CH3CN·χ(solvent)]n-(3) (where L1 and L2 represent 1,3,5-tris((pyridin-4-ylthio)methyl)benzene and 2,4,6-trimethoxy-1,3,5-tris((pyridin-4-ylthio) methyl)benzene; DMA = Dimethylacetamide) to utilize them for effective removal of Hg2+ in water [166]. These MOFs removed 90% of Hg2+within 5 min at optimum pHs 4 and 5. The maximum adsorption capacities of MOFs (1), (2), and (3) were estimated to be 362.3 mg g−1, 227.4 mg g−1, and 341.7 mg g−1, respectively. The observed higher efficiencies to Hg2+was attributed to the strong binding between Hg-S. They were reusable up to five cycles (with Na2S). This work followed the pseudo-second-order model and was linearly correlated with the Langmuir isotherm. This work is considered a good one because of the negligible interference effect, but further optimization is required to improve the adsorption capacity.
The bi-metallic MOFs were also employed in Hg2+ removal/extraction as described next. Han and co-workers constructed the heterometallic metal−organic framework (HMOF): {[(CH3)2NH2]InCu4L4·xS}n, namely BUT-52 (where L represents 6,6′-dithiodinicotinic acid), to engage in Hg2+ removal, in which In(COO)4 and Cu6S6 clusters were rationally embedded [167]. The BET surface area of BUT-52 to N2 gas at 77K was 126.2 cm3 g−1. It showed 92% of mercury removal efficiency in ethanol. This work requires further optimization in anti-interference, pH, time, and real time application studies. Mon et al. described utilization of the Ca and Cu-based porous bimetallic MOF, namely {CaIICuII6[(S,S)-methox]3(OH)2(H2O)}·16H2O (where methox represents bis[(S)-methionine]oxalyl diamide), for Hg2+ and CH3Hg+ removal in aqueous media [168]. This BioMOF reduced Hg2+ and CH3Hg+ contents from 10 ppm to 5 and 27 ppb, respectively, with corresponding adsorbing ability of 99.95% and 99.0% (via Hg–S interactions) for dissolved HgCl2 and CH3HgCl salts. However, optimization is required to study the adsorption kinetics and real applications. In parallel with MOFs-based extraction/removal of Hg2+, a few MOFs were also reported in multiple heavy metal ions, including Hg2+ [169,170,171,172,173]. Though those reports demonstrated effective removal of Hg2+, but they were also affected by interfering effects from other ions. To avoid the interfering effects, complicated masking procedure is required. Therefore, those reports will not be discussed in this review.
9. MOFs Comprised Composites for Hg2+ Removal
Compared to MOFs, composites comprised of MOFs also become effective in Hg2+ removal [174]. For example, the Pt NPs encapsulated UIO-66-NH2-(denoted as Pt NP@UIO-66-NH2) was engaged in facile colorimetric detection and removal of Hg2+ in water [175]. The Pt NP@UIO-66-NH2 displayed a linear colorimetric response from 0 to 10 nM with a LOD 0.35 nM due to the peroxidase like activity which took place in the presence of 3,3′,5,5′-tetramethylbenzidine and H2O2. Moreover, the Pt NP@UIO-66-NH2was used as an adsorbent for Hg2+ with a maximum adsorption capacity of 206.25 mg g−1. This material showed Hg2+ removal of 99% and reduced Hg2+ concentrations from 5 ppm to 2.39 ppb. Reusability of this composite was established up to four cycles by Na2S. This work followed the pseudo-second-order model and was linearly fitted with the Langmuir isotherm. With respect to multiple applications, this work is considered a nice one. The mercapto-functionalized Zr-MOF/melamine sponge composite (Zr-MOF-SH/MF) was proposed for removal of Hg2+ from water [176]. Firstly, zirconium chloride (ZrCl4) was reacted with meso-tetra(4-carboxyphenyl)porphine (H2TCPP). The product was then interacted with mercaptoacetic acid to afford PCN-224-MAA, which formed the composite structure with melamine sponge. In particular, the PCN-224-MAA/MF (where MAA and MF represent mercaptoacetic acid and melamine formaldehyde) showed great ability for Hg2+ removal (among other heavy metal ions) with a maximum adsorption capacity of 412.5 mg g−1 at pH 3 as seen in Figure 12. Due to the Hg–S interactions, the removal process was found to be efficient and effective. The presence of melamine sponge extended the Hg2+ adsorption cycle up to five times. This work showed more effectiveness in Hg2+ removal from water-oil mixture, but the underlying kinetics require further studies.
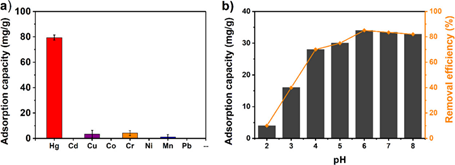
Figure 12. (a) Adsorption of mixed heavy metal ions (concentration of each ion was 100 mg L−1 on the PCN-224-MAA/MF composite at pH 3. (b) Effect of pH on the removal of Hg2+ ions with an initial concentration of 20 mg L−1 on a PCN-224-MAA/MF (Reproduced with the permission from Ref [176]).
Novel ZnS-ZIF-8 monolith was explored in Hg2+ capture in wastewater [177]. The ZIF-8 filter paper was first developed by reacting Zn (NO3)2·6H2O with 2-methylimidazole followed by sulfurization to obtain the ZnS-ZIF-8 monolith, which possessed a hierarchical porous crystalline structure. At an optimum value of pH 5, the monolith showed a maximum adsorption capacity of 925.9 mg g−1 with recyclability with Na2S. The efficiency was found to be high because of the effective Hg–S interaction. This work followed the pseudo-second-order kinetic and was correlated with Langmuir isotherm. This work is considered a nice one in terms of its selectivity and applicability. Nosike and co-workers proposed utilization of the Fe3O4@ZIF-90-Cysteine composite towards Hg2+ adsorption as detailed below [178]. Wherein, the Fe3O4 was first embedded into the ZIF-90 (a Zn-based zeoliticimidazolate framework) as a core. Cysteine was then covalently attached to the Fe3O4@ZIF-90 via Schiff base reaction and post-synthetic modification strategy to obtain the Fe3O4@ZIF-90-Cysteine. Poly-acrylic acid (PAA) was capped on the above composite to avoid agglomeration of nanoparticles. Because of the pore size and free thiol (-SH) group of cysteine, this composite showed good adsorbing ability to Hg2+ with a maximum capacity of 900 mg g−1 at optimal pH 4. The adsorption followed the pseudo-second-order model. Desorption and regeneration were accomplished with HCl (0.1 M) up to four cycles. However, the synthetic complications may affect its applicability. Huang et al. described the magnetic MOF composite, namely bi-I-functionalized Fe3O4@SiO2@HKUST-1 (where HKUST-1 represents Cu-based MOF), with core–shell nanostructures for enhanced removal of Hg2+ in water [179]. The bismuthiol I (1,3,4-thiadiazole-2,5-dithiol, Bi-I) was functionalized over the Fe3O4@SiO2@HKUST-1 via post-synthetic modification. As shown in Figure 13, the bi-I-functionalized Fe3O4@SiO2@HKUST-1 displayed high adsorption ability to Hg2+ with a maximum capacity of 264 mg g−1 between pHs 2–9. This work followed the pseudo second order model and was linearly fitted with the Langmuir isotherm. In both composites, the Fe3O4 was used to afford the magnetic property and played a vital role to improve the adsorption capacity. Performance of the bi-I-functionalized Fe3O4@SiO2@HKUST-1 was effective in the presence of other competing species. However, the main drawback of this study is that it cannot be reused with any eluent because of the strong Hg–S bond and the hydrolysis of Bi-I. Thereby this material can only be consumed for one-time removal of Hg2+ ions in water.
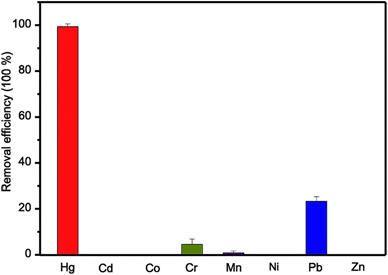
Figure 13. Selectivity of bi-I-functionalized Fe3O4@SiO2@HKUST-1 for Hg2+ conditions: each ion of 10 mg L−1 and pH 3 (reproduced with the permission from Ref [179]).
Liang and co-workers reported incorporation of In2S3 nanoparticles into the MIL-101 (a Cr-based MOF) to afford the In2S3@MIL-101 for effective removal of Hg2+ with an efficiency of 99.95% within a minute [180]. The BET surface area of the In2S3@MIL-101 to N2 gas at 77K was 1476 m2 g−1 with a maximum adsorption capacity of 518.2 mg g−1 for Hg2+. This work followed the pseudo second order kinetic model and was linearly correlated with the Langmuir isotherm. The strong affinity between Hg2+ to S was the main reason for high efficiency of the In2S3@MIL-101, which was able to reduce Hg2+ concentrations from 10 ppm to 1.75 ppb. Moreover, the composite operated between pHs 3 to 8 and was effective in the presence of competing species. By using 0.1 M KCl solution, the materials can be recycled up to three times, thereby is noted a remarkable work. In fact, incorporation of In2S3 nanoparticles improves the adsorption ability of MIL-101 to Hg2+ions. Similar to selective removal of Hg species, the MOF containing composites were also engaged in adsorption and capture of Pb2+ [181,182,183]. However, those reports will not be discussed in this review due to the possible interference effects.
10. MOFs and its Analogous in Elemental Mercury (Hg0)Adsorption
Due to the high toxic effect of elemental mercury (Hg0) from flue gas, its adsorption and separation is in high demand. Moreover, feasible utilization of MOFs and its analogous have been predicted theoretically [184,185]. For example, Zhao et al. reported Hg0 removal from flue gas of iron and steel by using the MIL-101(Cr). Performance of the MIL-101(Cr) was compared to the Cu-BTC (BTC represents benzene-1,3,5-tricarboxylate), UIO-66, and activated carbon [186]. Wherein, Hg0 was primarily adsorbed over the surface of MIL-101(Cr) and was oxidized by the open metal site Cr3+. Be noted that the MIL-101(Cr) captured 88% of Hg0 at 250 °C and also showed good thermal and chemical stability. Exceptional adsorption ability of the MIl-101(Cr) was further demonstrated by Dong and co-workers through Hg0 removal from the coal-fired boiler flue gas experiment [187]. Simulation studies also agreed with the experimental results, in which Hg0 adsorption over the MIL-101(Cr) surface followed the pseudo-second-order model as shown in Figure 14. Moreover, the MIl-101(Cr) showed the Langmuir-type rate expression with an estimated equilibrium adsorption capacity for the MOF-sorbent of 25.656 μg g−1 at 200 °C. Thus, the MIL-101(Cr) is considered a potential candidate for Hg0 removal.
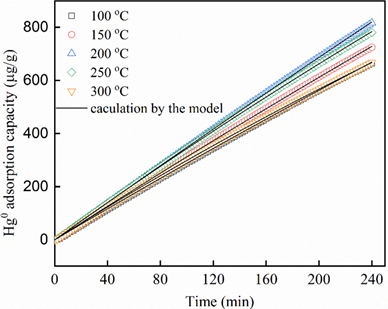
Figure 14. Simulation of Hg0 adsorption on MIL-101(Cr) by the pseudo-second-order model. Reaction conditions: N2 + 5% O2, inlet concentration of Hg0 = 203 μg m−3, gas hourly space velocity (GHSV) = 8 × 105 h−1, 200 °C (reproduced with the permission from Ref [187]).
Utilization of the Cu-BTC (BTC represents benzene-1,3,5-tricarboxylate) for Hg0 removal from sintering gas was discussed by Chen and co-workers [188]. The Cu-BTC showed great efficiency in the presence of 15 ppm HCl with the combination of O2. The chemisorbed O2 (over Cu2+ surface) oxidized the Hg0to Hg2+, which was then converted to HgCl2 in the presence of HCl. Moreover, the inhibition effect of SO2 was overcome by HCl, thereby the Cu-BTC is noted a material with good performance for Hg0 adsorption. The MnOxloaded on the MIL-100(Fe) (77.4% efficiency at 250 °C; GHSV = 18,000 h−1) and α-MnO2anchored MIL-96(Al) were reported for Hg0 removal from flue gas [189,190]. Because the presence of both MnOx and α-MnO2 can enhance adsorption of Hg0 and oxidation process, therefore, such composited MOFs can be engaged for environmental remediation. Subsequently, Yang et al. reported the nanosized CuSe functionalized Zn-comprising zeolitic imidazolate framework-8 (CuSe/ZIF-8) for Hg0 adsorption and removal studies [191]. The as-prepared CuSe/ZIF-8 with the 80% mass ratio of CuSe to ZIF-8 (to afford 0.8NC-ZIF) displayed an equilibrium Hg0 adsorption capacity with an average rate of 309.8 mg g−1 and 105.3 µg g−1 min−1, correspondingly, which were far better than that of the reported metal sulfide and activated carbon sorbents. The HgSe, which is more stable than HgS, can be easily formed because of the strong affinity between Hg and Se2- (from CuSe). The composite can be used for continuous removal of Hg0, thereby is considered an exceptional material. Following the similar approach, the Se functionalized MIL-101-Cr (Se/MIL-101-Cr) was proposed for Hg0 removal [192]. By means of stable and water-insoluble HgSe formation, the Se/MIL-101-Cr showed a maximum Hg adsorption capacity of 48.19 mg g−1, which was far better than commercially activated carbon. Moreover, the adsorption rate reached a value of 44.8 μg g−1 min−1 and became more-effective in flue gas atmosphere containing SO2, NO, and H2O. Thus, it is noted as a nice candidate for Hg0 sorption.
Zhao and co-workers reported Hg0 removal by combining Ag NPs with the Zr-based MOF-UIO-66 [193]. The adsorption capacity reached a value of 3.7 mg g−1 at 50oC because of the significant synergistic effect of Ag NPs over the UIO-66. Removal of Hg0 was attributed to the Ag amalgam formation and channel adsorption at low temperature. At high temperature, removal of Hg0 was driven by the Ag-activated oxygen oxidation and channel capture. Zhang et al. proposed the consumption of the Mn–Ce loaded MOF (MnCe@MOF) for removal of Hg2+ and NO from flue gas at low temperature [194]. However, this study reported a possible interfering effect of NO over Hg0 adsorption.
11. MOFs for Simultaneous Detection and Removal of Hg2+
As suggested by earlier studies [153,175], MOFs were also engaged in simultaneous detection and removal studies as discussed in this section. For instance, Rudd et al. demonstrated heavy metal ions sensing and removal using solvothermally synthesized Zn-based luminescent MOFs, namely Zn2(ofdc)2(tppe)-LMOFs-261, Zn2(hfdc)2(tppe)-LMOFs-262, and Zn2(dbtdcO2)2(tppe)-LMOFs-263), where H2ofdc, H2dbtdcO2, and tppe represent [9-oxo-9H-fluorene-2,7-dicarboxylic acid], [dibenzo[b,d]thiophene-3,7-dicarboxylic acid-5,5-dioxide], and 1,1,2,2-tetrakis(4-(pyridine-4-yl)phenyl)ethane, respectively [195]. Among these MOFs, the LMOFs-263 displayed the highest luminescent selectivity to Hg2+ and Pb2+with LODs of 3.3 and 19.7 ppb, respectively. Moreover, it showed a maximum adsorption capacity of 380 mg g−1 (for Hg2= within 30 min) and the adsorption followed pseudo-second-order kinetics. A Kd value of 6.45 × 105 mL g−1 was determined for the LMOFs-263. The effective adsorption was attributed to the strong interaction between Hg2+and SO22- (of H2dbtdcO2) and the pore size. The BET surface area of LMOFs-263 was estimated as 1004 m2 g−1 to N2 gas at 77K, however, further investigations are required to overcome the Pb2+ interference. Al-based imidazolate framework, namely NH2-MIL-53(Al), for selective detection and removal of Hg2+ was reported by Zhang and co-workers [196]. Because of the coordination of amino (-NH2) group and ligand-to-metal charge transfer (LMCT) effect with Hg2+, fluorescent intensity of the NH2-MIL-53(Al) at 427 nm (λex = 330 nm) was linearly quenched between 1−17.3 μM with a LOD of 0.15 μM. In addition, Hg2+ sensing ability of the NH2-MIL-53(Al) was good at pHs 4–10 without any interference. The MOF showed an adsorption capacity of 53.85 mg g−1 (for Hg2+) and was reusable with 0.1 M HCl and 10% thiourea eluent. This work followed pseudo second order kinetic model and was linearly correlated with the Langmuir isotherm, thereby is a nice probe.
By loading the (bis(4-(dimethylamino)phenyl)methanethione) probe over the Al-based MOF (which was solvothermally synthesized by reacting Al(NO3)3·9H2O and terephthalic acid), detection and removal of Hg2+ in water and skin-whitening cosmetics was delivered by Radwan and co-workers [197]. These thioketone Al-MOFs monitors (TAM) acted as microporous carriers towards Hg2+ via fluorescent quenching at 470 nm and enhancement at 610 nm with a linear range from 2 nM to 2.1 µM and a LOD of 4.4 nM. Moreover, the thioketone Al-MOF (TAM) nanorods were used in effective adsorption of Hg2+, which showed a maximum adsorption capacity of 1110 mg g−1 with exceptional applicability in water and skin-whitening cosmetics. Shahat et al. engaged the modified amino-functionalized Al-MOF for optical recognition and removal of Hg2+ [198]. AlCl3·6H2O and 2-amino terephthalic acid was first solvothermally reacted to yield the MOF-NH2-MIL-101(Al) followed by modification with ninhydrin to obtain the final adduct Nin-NH-MIL-101(Al). The Nin-NH-MIL-101(Al) showed a BET surface area of 896.6 m2 g−1 for N2 gas at 77K. It was used as a colorimetric sensory probe for Hg2+ with a LOD of 0.494 µg L−1 and was further applied in removal studies. The probe displayed a maximum adsorption capacity of 127.4 mg g−1 and was recyclable in the presence of 0.1 M thiourea as shown in Figure 15. This work followed the pseudo second order kinetic model and was linearly fitted Langmuir isotherm. Be noted that the Nin-NH-MIL-101(Al)-based optical detection and removal of Hg2+ was not affected by any interference.
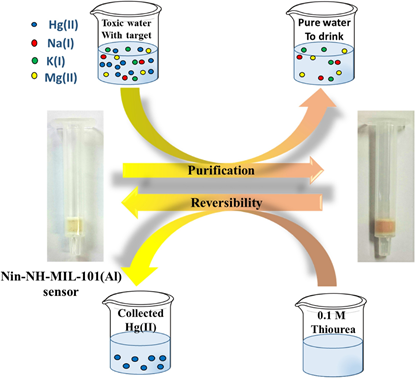
Figure 15. Representative design of the Nin-NH-MIL-101(Al) sensor applied to purification of water polluted with Hg(II) ions and the reversible process by using 0.1 M thiourea solution for several times (reproduced with the permission from Ref [198]).
By means of hydrothermal reactions, the Cu-based MOFs and amide-functionalized pillar ligands (–NH–CO–), namely TMU-46, 47, and 48, were synthesized and then decorated with suitable functional group malonamide (S) to produce the labelled dual functionalized materials-TMU-46S, TMU-47S, and TMU-48S, respectively. They were applied towards Hg2+ sensing and removal [199]. The BET surface areas of TMU-46S, TMU-47S, and TMU-48S were 510 m2 g−1, 520 m2 g−1, and 408 m2 g−1, respectively. Because of the strong coordination of Hg to S, the TMU-48S displayed the highest selectivity to Hg2+ via fluorescent quenching at 480 nm (λex = 330 nm) with a KSV value of 86,087 M−1. Moreover, the TMU-48S showed a maximum adsorption capacity of 714 mg g−1. However, it also showed some selectivity to Pb2+ and Ag+. The CuS particles (PCuS) were synthesized via wet-treatment of Cu-based MOF- HKUST-1 and were engaged in colorimetric detection of Hg2+ in the presence of 3,3′,5′,5-tetramethylbenzidine (TMB) and H2O2 (by peroxidase like activity) [200]. The BET surface area of PCuS was calculated to be 35 m2 g−1 with a linear colorimetric response between 3–40 µM and an established LOD of 0.22 µM. Moreover, the PCuS showed a maximum adsorption capacity of 2105 mg g−1. The system followed the pseudo second order kinetic and was linearly fitted with the Langmuir isotherm.
A porphyrinic Zr-based MOF, namely PCN-221 (by solvothermal reaction between meso-tetra(4-carboxyphenyl) porphyrin (TCPP) and ZrCl4), was proposed for fluorescent sensing and removal of Hg2+ in water [201]. The PCN-221 showed linear quenching at 436 nm (λex = 280 nm) in the presence of Hg2+ concentrations from 0 to 300 μM with a KSV value of 4021.9 M−1 and a LOD of 0.01 μM. Moreover, sensing ability of DMF by PCN-221 was also described in this report with extensive Hg2+ adsorption studies. The PCN-221 displayed a maximum capacity of 233.65 mg g−1 towards Hg2+ adsorption and was highly effective at pH 7. Three adsorption-desorption cycles were achieved in the presence of 0.2 M Na2EDTA without any interference effect. This study followed the pseudo second order model and was linearly correlated by the Langmuir isotherm. Recently, a 3D-microporous carbon/Zr-2,5-dimercaptoterephthalic acid MOFs (Zr-DMBD MOFs/3D-KSC) nanocomposite was delivered towards electrochemical detection and removal of Hg2+ [202]. Sensitivity of the nanocomposite to Hg2+ was established as 24.58 μA μM−1 cm−2 with a linear range of 0.25–3.5 μM and a LOD of 0.05 µM. It was confirmed that specificity and effectiveness of the composite were similar to sensory and other utilities of nanomaterials [203,204,205,206]. This may be due to the presence of thiol (-SH) group of 2,5-dimercaptoterephthalic acid, which has great affinity to Hg2+. The Zr-DMBD MOFs/3D-KSC showed a maximum adsorption capacity (for Hg2+) of 19.3 ± 0.52 mg g−1 (within 60 min at pH 6) and was reusable up to five cycles with EDTA. This work was also applied in real water samples. However, information regarding adsorption kinetics still needs to be discussed. Apart from specific Hg2+ sensing/adsorption utilization of MOFs, the Hg-metalated MOF scaffold can be employed for detection of other species. For example, Hg-metalated PCN-222 was reported as fluorescent and visual sensors for cysteine [207], which pointed out the possible future direction of MOFs-based Hg2+ sensors.
12. Advantages
Consumption of MOFs and their analogous for selective Hg2+ detection and removal possess many advantages and some restrictions as follows.
-
The majority of MOFs and their derivatives detect or adsorb the Hg species in aqueous media, therefore, MOFs-based detection and removal experiments could sustain the eco-friendly process via decontaminating the toxic mercury from aquatic environment.
-
Due to the porous nano/micro structural features, MOFs can be tuned towards encapsulation of specific Hg species, which could be further enhanced by post-synthetic modification or loading of specific groups, such as thiols (-SH).
-
MOFs and their analogous have the advantage of recognition of multiple analytes, including Hg2+, via variations of detection conditions, masking agents, and analyte concentrations.
-
MOFs can act as probes towards recognition and removal of Hg species through many tactics, such as optical, electrochemical, photoelectrochemical, etc. Thus, they are noted as materials with exceptional advantages.
-
By tuning the compositions to adjust the specific porous surface, many composites comprised of MOFs have unique advantages of capturing Hg species in the presence of other interfering analytes.
-
MOFs mediated Hg detection/removal process can be further extended towards recognition of specific bio-analytes, such as glutathione, cysteine, and thiol containing species.
13. Limitations
-
Synthesis of designated MOFs and their analogous is still considered a tough task due to certain limitations, such as possible co-adduct formation, suitable tactics, reaction conditions, solvent, etc.
-
Though MOFs display high sensitivity via fluorescence quenching or enhancement, however, many of them are consisted of toxic metals, such as Al, Cr, Zr, Lanthanides, etc. Therefore, bioimaging or biological assays of Hg2+ by these MOFs are restricted.
-
MOFs with free thiol (-SH) containing organic linkers also showed selectivity to Pb2+, Cd2+, and Ag+, thereby limiting high selectivity towards Hg species via certain interfering effect.
-
Majority of MOFs-based Hg2+ adsorption or removal studies were limited by many factors, such as MOFs concentration, structural stability, porosity, pH, time, operating temperature, suitable eluent, etc. Those factors require further attention.
-
Design and development of certain MOFs comprised composites are also limited by the multiple complicated procedures, which not only increase the cost of the processes but also restrict the commercialization of materials.
-
Complete characterizations of the Hg assay and removal processes also requires many costly instruments, such as scanning electron microscopy (SEM), powder-X-ray diffraction analyzer (PXRD), elemental analyzer, thermogravimetric analyzer (TGA), etc., which limits future research towards development of MOFs-based materials for mercury remediations.
-
Finally, adsorption capacities of a few MOFs were found to be affected by the multiple interference effects and physical/chemical stability of MOFs during the Hg assays in real samples. Thus, much focus is anticipated to address this problem.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors9050101
References
- Yee, K.-K.; Reimer, N.; Liu, J.; Cheng, S.-Y.; Yiu, S.-M.; Weber, J.; Stock, N.; Xu, Z. Effective Mercury Sorption by Thiol-Laced Metal–Organic Frameworks: In Strong Acid and the Vapor Phase. J. Am. Chem. Soc. 2013, 135, 7795–7798.
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanoca-talysis. Chem. Rev. 2020, 120, 1438–1511.
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53.
- Lee, K.J.; Lee, J.H.; Jeoung, S.; Moon, H.R. Transformation of Metal–Organic Frameworks/Coordination Polymers into Functional Nanostructured Materials: Experimental Approaches Based on Mechanistic Insights. Acc. Chem. Res. 2017, 50, 2684–2692.
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41.
- Hu, M.-L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal–organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598–1632.
- Dalapati, R.; Biswas, S. Post-synthetic modification of a metal-organic framework with fluorescent-tag for dual naked-eye sensing in aqueous medium. Sens. Actuators B Chem. 2017, 239, 759–767.
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55.
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Zhao, W.; Gu, J. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase. RSC Adv. 2016, 6, 69807–69814.
- Zhang, X.; Xia, T.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. Highly sensitive and selective detection of mercury (II) based on a zirconium metal-organic framework in aqueous media. J. Solid State Chem. 2017, 253, 277–281.
- Samanta, P.; Desai, A.V.; Sharma, S.; Chandra, P.; Ghosh, S.K. Selective Recognition of Hg2+ ion in Water by a Functionalized Metal–Organic Framework (MOF) Based Chemodosimeter. Inorg. Chem. 2018, 57, 2360–2364.
- Xiaoxiong, Z.; Wenjun, Z.; Cuiliu, L.; Xiaohong, Q.; Chengyu, Z. Eu3+-Postdoped UIO-66-Type Metal-Organic Framework as a Luminescent Sensor for Hg2+ Detection in Aqueous Media. Inorg. Chem. 2019, 58, 3910–3915.
- Wang, Z.; Yang, J.; Li, Y.; Zhuang, Q.; Gu, J. Zr-Based MOFs integrated with a chromophoric ruthenium complex for specific and reversible Hg2+sensing. Dalton Trans. 2018, 47, 5570–5574.
- Li, W.-Y.; Yang, S.; Li, Y.-A.; Li, Q.-Y.; Guan, Q.; Dong, Y.-B. Synthesis of an MOF-based Hg2+-fluorescent probe via stepwise post-synthetic modification in a single-crystal-to-single-crystal fashion and its application in bioimaging. Dalton Trans. 2019, 48, 16502–16508.
- Kim, J.; Oh, J.S.; Park, K.C.; Gupta, G.; Lee, C.Y. Colorimetric detection of heavy metal ions in water via metal-organic framework. Inorg. Chim. Acta 2019, 486, 69–73.
- Wang, H.; Wang, X.; Liang, M.; Chen, G.; Kong, R.-M.; Xia, L.; Qu, F. A Boric Acid-Functionalized Lanthanide Metal-Organic Framework as a Fluorescence “Turn-on” Probe for Selective Monitoring of Hg2+ and CH3Hg+. Anal. Chem. 2020, 92, 3366–3372.
- Xia, T.; Song, T.; Zhang, G.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. A Terbium Metal-Organic Framework for Highly Selective and Sensitive Luminescence Sensing of Hg2+ Ions in Aqueous Solution. Chem. A Eur. J. 2016, 22, 18429–18434.
- Liu, X.; Lin, H.; Xiao, Z.; Fan, W.; Huang, A.; Wang, R.; Zhang, L.; Sun, D. Multifunctional lanthanide–organic frameworks for fluorescent sensing, gas separation and catalysis. Dalton Trans. 2016, 45, 3743–3749.
- Razavi, S.A.A.; Masoomi, M.Y.; Morsali, A. Double Solvent Sensing Method for Improving Sensitivity and Accuracy of Hg(II) Detection Based on Different Signal Transduction of a Tetrazine-Functionalized Pillared Metal-Organic Framework. Inorg. Chem. 2017, 56, 9646–9652.
- Wan, Y.; Zou, D.; Cui, Y.; Yang, Y.; Qian, G. A Zn based anionic metal-organic framework for trace Hg2+ ion detection. J. Solid State Chem. 2018, 266, 70–73.
- Pankajakshan, A.; Kuznetsov, D.; Mandal, S. Ultrasensitive Detection of Hg(II) Ions in Aqueous Medium Using Zinc-Based Metal-Organic Framework. Inorg. Chem. 2019, 58, 1377–1381.
- Khatun, A.; Panda, D.K.; Sayresmith, N.; Walter, M.G.; Saha, S. Thiazolothiazole-Based Luminescent Metal-Organic Frameworks with Ligand-to-Ligand Energy Transfer and Hg2+-Sensing Capabilities. Inorg. Chem. 2019, 58, 12707–12715.
- Jiang, J.; Lu, Y.; Liu, J.; Zhou, Y.; Zhao, D.; Li, C. An acid-base resistant Zn-based metal-organic framework as a luminescent sensor for mercury(II). J. Solid State Chem. 2020, 283, 121153.
- Wu, P.; Liu, Y.; Liu, Y.; Wang, J.; Li, Y.; Liu, W.; Wang, J. Cadmium-Based Metal-Organic Framework as a Highly Selective and Sensitive Ratiometric Luminescent Sensor for Mercury(II). Inorg. Chem. 2015, 54, 11046–11048.
- Liu, B.-H.; Liu, D.-X.; Yang, K.-Q.; Dong, S.-J.; Li, W.; Wang, Y.-J. A new cluster-based metal-organic framework with triazine backbones for selective luminescent detection of mercury(II) ion. Inorg. Chem. Commun. 2018, 90, 61–64.
- El Taher, B.J.; Sabouni, R.; Ghommem, M. Luminescent metal organic framework for selective detection of mercury in aqueous media: Microwave-based synthesis and evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125477.
- Basaleh, A.S.; Sheta, S.M. Novel advanced nanomaterial based on ferrous metal-organic framework and its application as chemosensors for mercury in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 3153–3165.
- Li, Y.; Ma, D.; Chen, C.; Chen, M.; Li, Z.; Wu, Y.; Zhu, S.; Peng, G. A hydrostable and bromine-functionalized manganese-organic framework with luminescence sensing of Hg2+ and antiferromagnetic properties. J. Solid State Chem. 2019, 269, 257–263.
- Song, Y.; Fan, R.; Fan, J.; Xing, K.; Du, X.; Wang, P.; Yang, Y. Highly sensitive and selective fluorescent probes for Hg2+ in Ag(i)/Cu(ii) 3D supramolecular architectures based on noncovalent interactions. Dalton Trans. 2016, 45, 16422–16432.
- Jana, A.K.; Natarajan, S. Fluorescent Metal-Organic Frameworks for Selective Sensing of Toxic Cations (Tl3+, Hg2+) and Highly Oxidizing Anions ((CrO4)2−, (Cr2O7)2−, (MnO4)−). ChemPlusChem 2017, 82, 1153–1163.
- Xiao, J.; Liu, J.; Gao, X.; Ji, G.; Wang, D.; Liu, Z. A multi-chemosensor based on Zn-MOF: Ratio-dependent color transition detection of Hg (II) and highly sensitive sensor of Cr (VI). Sens. Actuators B Chem. 2018, 269, 164–172.
- Fu, H.-R.; Zhao, Y.; Xie, T.; Han, M.-L.; Ma, L.-F.; Zang, S.-Q. Stable dye-encapsulated indium-organic framework as dual-emitting sensor for the detection of Hg2+/Cr2O72− and a wide range of nitro-compounds. J. Mater. Chem. C 2018, 6, 6440–6448.
- Li, F.; Hong, Y.-S.; Zuo, K.-X.; Sun, Q.; Gao, E.-Q. Highly selective fluorescent probe for Hg2+ and MnO4− by the two-fold interpenetrating metal-organic framework with nitro functionalized linkers. J. Solid State Chem. 2019, 270, 509–515.
- Yang, Y.-J.; Liu, D.; Li, Y.-H.; Dong, G.-Y. Two new luminescent ternary Cd(II)-MOFs by regulation of aromatic dicarboxylate ligands used as efficient dual-responsive sensors for toxic metal ions in water. Polyhedron 2019, 159, 32–42.
- Mahmoud, M.E.; Moussa, Z.; Prakasam, T.; Li, L.; Abiad, M.G.; Patra, D.; Hmadeh, M. Lanthanides based metal organic frameworks for luminescence sensing of toxic metal ions. J. Solid State Chem. 2020, 281, 121031.
- Ren, M.; Wang, H.; Liu, Y.; Ma, Q.; Jia, W.; Liu, M.; Wang, H.; Lu, Y. Fluorescent Determination of Mercury (II) and Glutathione Using Amino-MIL-53(Al) Nanosheets. Anal. Lett. 2020, 53, 2700–2714.
- Yang, L.; Li, X.; Qin, C.; Shao, K.-Z.; Su, Z.-M. A fluorescent sensor for highly selective sensing of nitro explosives and Hg(ii) ions based on a 3D porous layer metal–organic framework. CrystEngComm 2016, 18, 4765–4771.
- Gong, W.-J.; Ren, Z.-G.; Li, H.-X.; Zhang, J.-G.; Lang, J.-P. Cadmium(II) Coordination Polymers of 4-Pyr-poly-2-ene and Carboxylates: Construction, Structure, and Photochemical Double [2 + 2] Cycloaddition and Luminescent Sensing of Nitroaromatics and Mercury(II) Ions. Cryst. Growth Des. 2017, 17, 870–881.
- Su, Y.-Q.; Qu, Y.-H.; Fu, L.; Cui, G.-H. An unprecedented binodal (4,6)-connected Co(II) MOF as dual-responsive luminescent sensor for detection of acetylacetone and Hg2+ ions. Inorg. Chem. Commun. 2020, 118, 108013.
