The Neuropeptide S (NPS) system was discovered by a "reverse pharmacology" approach in search for the endogenous ligand of an orphan G protein-coupled receptor. Its peptide ligand and receptor are mainly found in the brain. Effects on anxiety and memory have been described for NPS, as well as genetic associations of the receptor gene with asthma and inflammatory diseases.
- transmitter
- GPCR
- brainstem
- anxiety
- memory
- genetic variation
1. Introduction
Neuropeptide S (NPS) was discovered as a ligand of a previously orphan G protein-coupled receptor (GPCR) by using the “orphan receptor strategy”, also known as “reverse pharmacology” [1]. The receptor (previously known as GPR154 or GPRA) was stably expressed in cells that served as a bait to purify the endogenous ligand from brain extracts [2]. The ligand turned out to be a peptide of 20 amino acids that contains a perfectly conserved serine (single amino acid code “S”) at the N-terminus in all species analyzed, and was thus termed accordingly [3]. NPS is encoded as a single copy peptide by a rather small precursor protein (<90 amino acids) that occurs in the genome of all tetrapods but is absent from fish [4]. The seven N-terminal residues are identical in all tetrapods, as well as the overall 20 amino acid length, while the C-terminal half shows more variation (Figure 1).
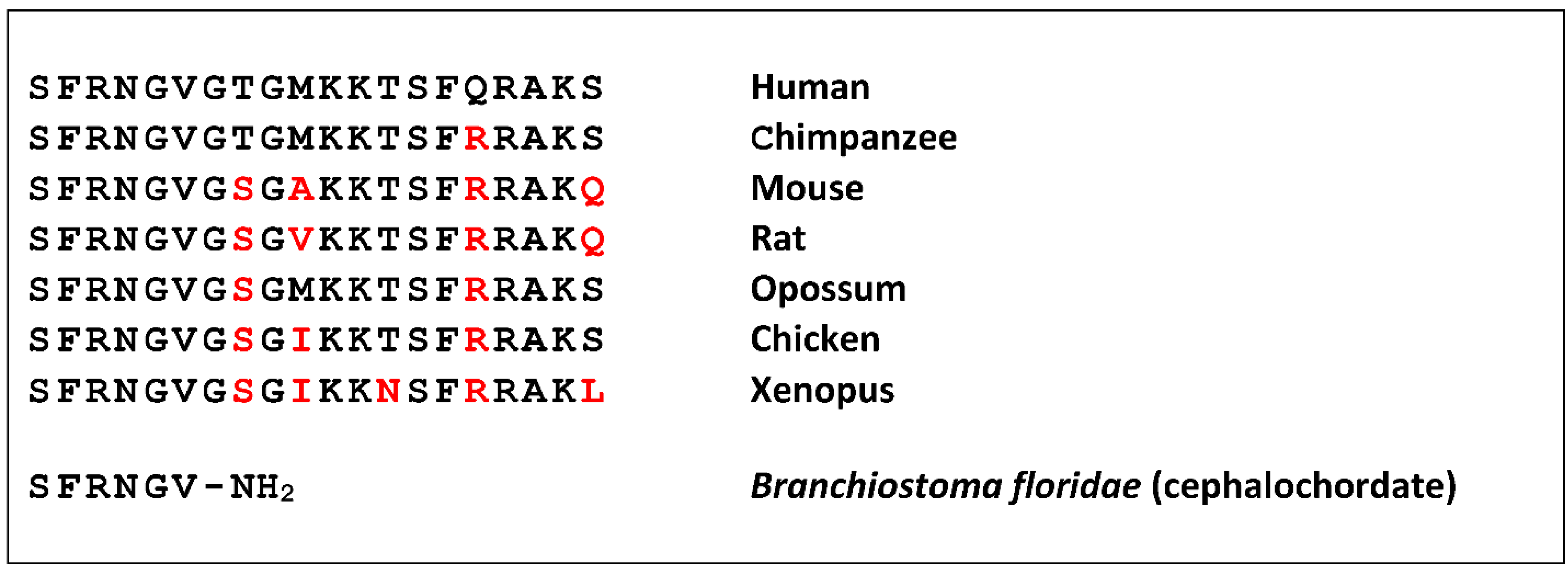
A shortened peptide has been identified in a cephalochordate that contains the conserved amino terminal residues [5]. Together with the identification of a distantly-related GPCR from the same class of animals, this suggests a rather complex evolution of the NPS system in bilaterians, where teleost fish may have lost both genes [6].
NPSR1 is also a single-copy gene with moderate similarity to other peptide GPCRs, the closest being vasopressin 1A and oxytocin receptors. NPSR1 is found in the genomes of all tetrapods and no convincing orthologues have been identified in fish genomes [7].
2. General Pharmacology
NPSR1 couples via Gαs and Gαq to elevate intracellular cAMP and Ca2+, thus it is an excitatory GPCR [8]. Activation of mitogen-activated protein kinase (MAPK) pathways and opening of neuronal Ca2+ channels have also been described [8][9][10]. NPS activates its receptor at low nanomolar concentrations and structure-activity relationship (SAR) studies have confirmed the importance of the amino terminus for agonist activity, overlapping with the high evolutionary conservation of this part of the peptide structure [11][12][13]. Focused SAR studies performed on Gly5 lead to the identification of peptidergic NPSR1 antagonists, characterized by a D-amino acid with a short branched aliphatic side chain [14][15]. Some well-characterized NPSR1 antagonists identified in the frame of these studies are [D-Cys(t-Bu)5]NPS, [D-Val5]NPS, and [t-Bu-D-Gly5]NPS [15][16][17].
The prototype synthetic NPSR1 antagonist is SHA 68, belonging to the class of diphenyltetrahydro-1H-oxazolo [3,4-α]pyrazines [18] (Figure 2). SHA 68, and the closely related SHA 66, display nanomolar affinity for NPSR1 in vitro but only limited bioavailability in vivo, due to its high lipophilicity. Using the core structure of SHA 68, several refinements have been made to improve in vivo potency. It appears that slight increases in polarity can indeed cause an increased in vivo potency, albeit with a net loss in receptor affinity [19][20][21][22]. Additional high-throughput drug screening programs have yielded further structures with NPSR1 antagonistic profiles (Figure 2), although with lower in vitro and in vivo potency compared to SHA 68 [23][24][25]. None of these compounds has progressed beyond the preclinical stage yet.
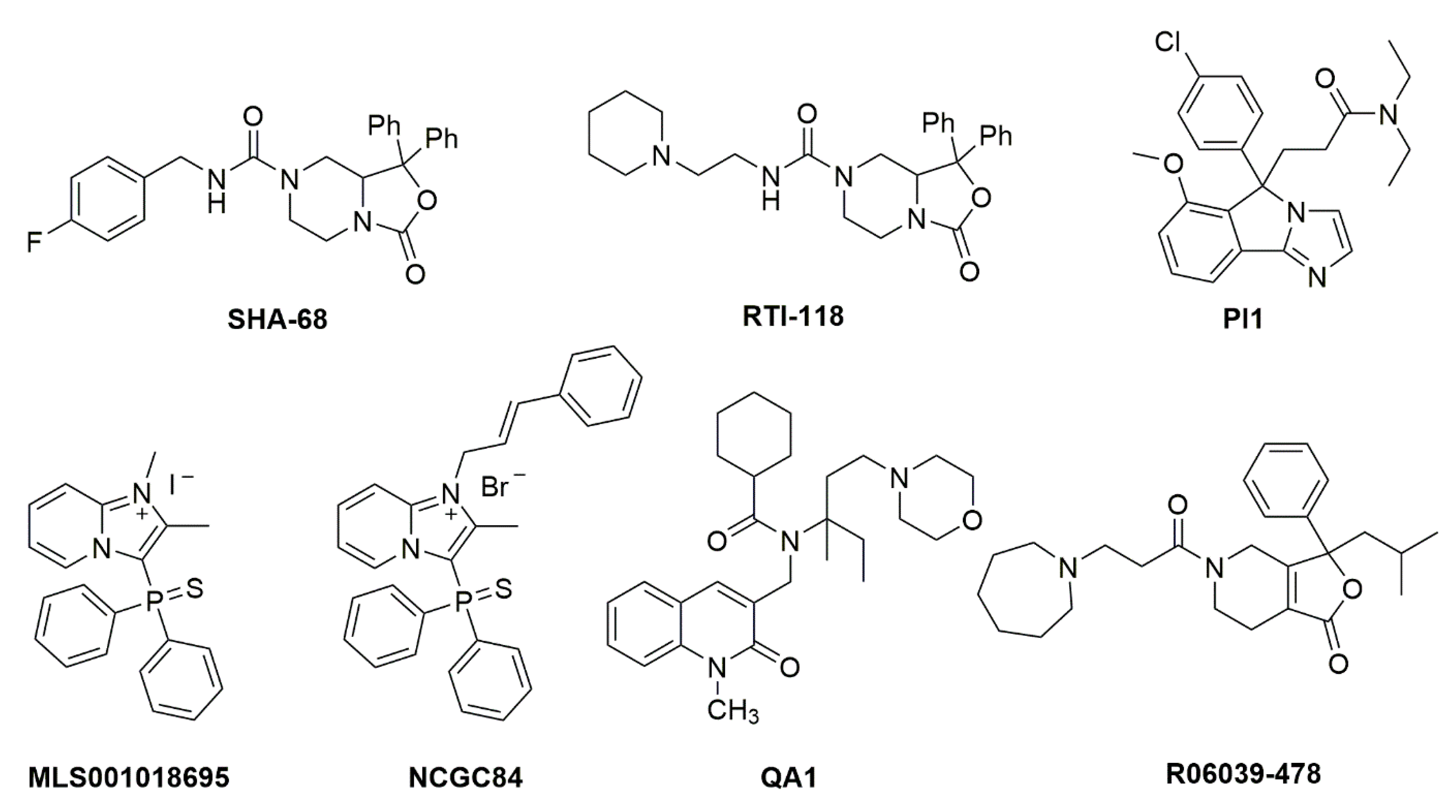
Figure 2. Chemical structures of NPSR1 antagonists. Details about synthesis and pharmacological activities can be found in the original literature. SHA 68 [18], RTI-118 [26], PI1 [27], MLS001018695 [28], NCGC84 [23], QA1 [29], R06039-478 [30].
3. Physiological and Behavioral Effects of NPS
3.1. Modulation of Animal Behavior
3.2. NPS and Immune Functions
4. Concluding Remarks
An impressive number of physiological functions have been identified for the NPS system in the relatively short time since its discovery. Together with the plethora of genetic association data for NPSR1 variants with human disease and behaviors, increased efforts to identify therapeutic applications for this interesting transmitter system appear to be promising and warranted. The articles in this Special Issue reflect the continuing progress in our knowledge about the NPS system and its therapeutic potential.
This entry is adapted from the peer-reviewed paper 10.3390/ph14050401
References
- Xu, Y.-L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; Brucher, F.A.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497.
- Rizzi, A.; Vergura, R.; Marzola, G.; Ruzza, C.; Guerrini, R.; Salvadori, S.; Regoli, D.; Calo, G. Neuropeptide S is a stimulatory anxiolytic agent: A behavioural study in mice. Br. J. Pharmacol. 2008, 154, 471–479.
- Leonard, S.K.; Dwyer, J.M.; Sukoff Rizzo, S.J.; Platt, B.; Logue, S.F.; Neal, S.J.; Malberg, J.E.; Beyer, C.E.; Schechter, L.E.; Rosenzweig-Lipson, S.; et al. Pharmacology of neuropeptide S in mice: Therapeutic relevance to anxiety disorders. Psychopharmacology 2008, 197, 601–611.
- Ionescu, I.A.; Dine, J.; Yen, Y.C.; Buell, D.R.; Herrmann, L.; Holsboer, F.; Eder, M.; Landgraf, R.; Schmidt, U. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology 2012, 37, 1323–1337.
- Dine, J.; Ionescu, I.A.; Avrabos, C.; Yen, Y.C.; Holsboer, F.; Landgraf, R.; Schmidt, U.; Eder, M. Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a “normal”-anxiety one. PLoS ONE 2015, 10, e0120272.
- Slattery, D.A.; Naik, R.R.; Grund, T.; Yen, Y.C.; Sartori, S.B.; Füchsl, A.; Finger, B.C.; Elfving, B.; Nordemann, U.; Guerrini, R.; et al. Selective breeding for high anxiety introduces a synonymous SNP that increases Neuropeptide S receptor activity. J. Neurosci. 2015, 35, 4599–4613.
- Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291.
- Okamura, N.; Garau, C.; Duangdao, D.M.; Clark, S.D.; Jüngling, K.; Pape, H.-C.; Reinscheid, R.K. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology 2011, 36, 744–752.
- Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405.
- Han, R.W.; Zhang, R.S.; Xu, H.J.; Chang, M.; Peng, Y.L.; Wang, R. Neuropeptide S enhances memory and mitigates memory impairment induced by MK801, scopolamine or Aβ1-42 in mice novel object and object location recognition tasks. Neuropharmacology 2013, 70, 261–267.
- Jüngling, K.; Seidenbecher, T.; Sosulina, L.; Lesting, J.; Sangha, S.; Clark, S.D.; Okamura, N.; Duangdao, D.M.; Xu, Y.-L.; Reinscheid, R.K.; et al. Neuropeptide S-Mediated Control of Fear Expression and Extinction: Role of Intercalated GABAergic Neurons in the Amygdala. Neuron 2008, 59, 298–310.
- Sartori, S.B.; Maurer, V.; Murphy, C.; Schmuckermair, C.; Muigg, P.; Neumann, I.D.; Whittle, N.; Singewald, N. Combined neuropeptide S and D-cycloserine augmentation prevents the return of fear in extinction-impaired rodents: Advantage of dual versus single drug approaches. Int. J. Neuropsychopharmacol. 2016, 19, 1–11.
- Okamura, N.; Reinscheid, R.K.; Ohgake, S.; Iyo, M.; Hashimoto, K. Neuropeptide S attenuates neuropathological, neurochemical and behavioral changes induced by the NMDA receptor antagonist MK-801. Neuropharmacology 2010, 58, 166–172.
- Smith, K.L.; Patterson, M.; Dhillo, W.S.; Patel, S.R.; Semjonous, N.M.; Gardiner, J.V.; Ghatei, M.A.; Bloom, S.R. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology 2006, 147, 3510–3518.
- Zhu, H.; Mingler, M.K.; McBride, M.L.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Williams, M.T.; Vorhees, C.V.; Rothenberg, M.E. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 2010, 35, 1119–1132.
- Si, W.; Aluisio, L.; Okamura, N.; Clark, S.D.; Fraser, I.; Sutton, S.W.; Bonaventure, P.; Reinscheid, R.K. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J. Neurochem. 2010, 115, 475–482.
- Li, W.; Chang, M.; Peng, Y.L.; Gao, Y.H.; Zhang, J.N.; Han, R.W.; Wang, R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul. Pept. 2009, 156, 90–95.
- Peng, Y.L.; Zhang, J.N.; Chang, M.; Li, W.; Han, R.W.; Wang, R. Effects of central neuropeptide S in the mouse formalin test. Peptides 2010, 31, 1878–1883.
- Victor Holanda, A.D.; Asth, L.; Santos, A.R.; Guerrini, R.; Soares-Rachetti, V.D.P.; Calo’, G.; André, E.; Gavioli, E.C. Central adenosine A1 and A2A receptors mediate the antinociceptive effects of neuropeptide S in the mouse formalin test. Life Sci. 2015, 120, 8–12.
- Holanda, V.A.D.; Oliveira, M.C.; Souza, L.S.; Lobão-Soares, B.; André, E.; Da Silva Junior, E.D.; Guerrini, R.; Calo, G.; Ruzza, C.; Gavioli, E.C. Dopamine D1 and D2 receptors mediate neuropeptide S-induced antinociception in the mouse formalin test. Eur. J. Pharmacol. 2019, 859, 172557.
- Lee, M.T.; Chiu, Y.T.; Chiu, Y.C.; Hor, C.C.; Lee, H.J.; Guerrini, R.; Calo, G.; Chiou, L.C. Neuropeptide S-initiated sequential cascade mediated by OX1, NK1, mGlu5 and CB1 receptors: A pivotal role in stress-induced analgesia. J. Biomed. Sci. 2020, 27, 7.
- Jinushi, K.; Kushikata, T.; Kudo, T.; Calo, G.; Guerrini, R.; Hirota, K. Central noradrenergic activity affects analgesic effect of Neuropeptide S. J. Anesth. 2018, 32, 48–53.
- Peng, Y.L.; Han, R.W.; Chang, M.; Zhang, L.; Zhang, R.S.; Li, W.; Han, Y.F.; Wang, R. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides 2010, 31, 2259–2263.
- Cifani, C.; Di Bonaventura, M.V.M.; Cannella, N.; Fedeli, A.; Guerrini, R.; Calo, G.; Ciccocioppo, R.; Ubaldi, M. Effect of neuropeptide S receptor antagonists and partial agonists on palatable food consumption in the rat. Peptides 2011, 32, 44–50.
- Didonet, J.J.; Cavalcante, J.C.; Souza, L.D.; Costa, M.S.; André, E.; Soares-Rachetti, V.D.; Guerrini, R.; Gavioli, E.C. Neuropeptide S counteracts 6-OHDA-induced motor deficits in mice. Behav. Brain Res. 2014, 266, 29–36.
- Cannella, N.; Economidou, D.; Kallupi, M.; Stopponi, S.; Heilig, M.; Massi, M.; Ciccocioppo, R. Persistent Increase of Alcohol-Seeking Evoked by Neuropeptide S: An Effect Mediated by the Hypothalamic Hypocretin System. Neuropsychopharmacology 2009, 34, 2125–2134.
- Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology 2016, 233, 2915–2924.
- Pañeda, C.; Huitron-Resendiz, S.; Frago, L.M.; Chowen, J.A.; Picetti, R.; De Lecea, L.; Roberts, A.J. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. 2009, 29, 4155–4161.
- Kallupi, M.; Cannella, N.; Economidou, D.; Ubaldi, M.; Ruggeri, B.; Weiss, F.; Massi, M.; Marugan, J.; Heilig, M.; Bonnavion, P.; et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. USA 2010, 107, 19567–19572.
- Kallupi, M.; De Guglielmo, G.; Cannella, N.; Li, H.W.; Caló, G.; Guerrini, R.; Ubaldi, M.; Renger, J.J.; Uebele, V.N.; Ciccocioppo, R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 2013, 226, 347–355.
- Ruzza, C.; Calò, G.; Di Maro, S.; Pacifico, S.; Trapella, C.; Salvadori, S.; Preti, D.; Guerrini, R. Neuropeptide S receptor ligands: A patent review (2005–2016). Expert Opin. Ther. Pat. 2017, 27, 347–362.
- Schmoutz, C.D.; Zhang, Y.; Runyon, S.P.; Goeders, N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012, 103, 332–337.
- Cannella, N.; Kallupi, M.; Ruggeri, B.; Ciccocioppo, R.; Ubaldi, M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013, 100, 48–59.
- Ubaldi, M.; Giordano, A.; Severi, I.; Li, H.; Kallupi, M.; De Guglielmo, G.; Ruggeri, B.; Stopponi, S.; Ciccocioppo, R.; Cannella, N. Activation of hypocretin-1/orexin-a neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol. Psychiatry 2016, 79, 452–462.
- Wegener, G.; Finger, B.C.; Elfving, B.; Keller, K.; Liebenberg, N.; Fischer, C.W.; Singewald, N.; Slattery, D.A.; Neumann, I.D.; Mathé, A.A. Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: A genetic animal model of depression. Int. J. Neuropsychopharmacol. 2012, 15, 375–387.
- Shirayama, Y.; Ishima, T.; Oda, Y.; Okamura, N.; Iyo, M.; Hashimoto, K. Opposite roles for neuropeptide S in the nucleus accumbens and bed nucleus of the stria terminalis in learned helplessness rats. Behav. Brain Res. 2015, 291, 67–71.
- Duangdao, D.M.; Clark, S.D.; Okamura, N.; Reinscheid, R.K. Behavioral phenotyping of Neuropeptide S receptor knockout mice. Behav. Brain Res. 2009, 205, 1–9.
- Fendt, M.; Buchi, M.; Bürki, H.; Imobersteg, S.; Ricoux, B.; Suply, T.; Sailer, A.W. Neuropeptide S receptor deficiency modulates spontaneous locomotor activity and the acoustic startle response. Behav. Brain Res. 2011, 217, 1–9.
- Liu, X.; Si, W.; Garau, C.; Jüngling, K.; Pape, H.-C.; Schulz, S.; Reinscheid, R.K. Neuropeptide S precursor knockout mice display memory and arousal deficits. Eur. J. Neurosci. 2017, 46, 1689–1700.
- Ruzza, C.; Pulga, A.; Rizzi, A.; Marzola, G.; Guerrini, R.; Calo’, G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 2012, 62, 1999–2009.
- Allen, I.C.; Pace, A.J.; Jania, L.A.; Ledford, J.G.; Latour, A.M.; Snouwaert, J.N.; Bernier, V.; Stocco, R.; Therien, A.G.; Koller, B.H. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1005–L1017.
- Zhu, H.; Perkins, C.; Mingler, M.K.; Finkelman, F.D.; Rothenberg, M.E. The role of neuropeptide S and neuropeptide S receptor 1 in regulation of respiratory function in mice. Peptides 2011, 32, 818–825.
- Pulkkinen, V.; Majuri, M.L.; Wang, G.; Holopainen, P.; Obase, Y.; Vendelin, J.; Wolff, H.; Rytilä, P.; Laitinen, L.A.; Haahtela, T.; et al. Neuropeptide S and G protein-coupled receptor 154 modulate macrophage immune responses. Hum. Mol. Genet. 2006, 15, 1667–1679.
- Yao, Y.; Su, J.; Yang, G.; Zhang, G.; Lei, Z.; Zhang, F.; Li, X.; Kou, R.; Liu, Y.; Liu, J. Effects of neuropeptide S on the proliferation of splenic lymphocytes, phagocytosis, and proinflammatory cytokine production of pulmonary alveolar macrophages in the pig. Peptides 2011, 32, 118–124.
- Yao, Y.; Su, J.; Zhang, F.; Lei, Z. Effects of central and peripheral administration of neuropeptide S on the level of serum proinflammatory cytokines in pigs. Neuroimmunomodulation 2014, 21, 45–51.
- Ilmarinen, P.; James, A.; Moilanen, E.; Pulkkinen, V.; Daham, K.; Saarelainen, S.; Laitinen, T.; Dahlén, S.E.; Kere, J.; Dahlén, B.; et al. Enhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100 IU/ml. Peptides 2014, 51, 100–109.
