1. Introduction
Interest in the oceans has grown in recent decades due to the enlargement of discovery in quantity and diversity of marine products with interesting biological/pharmacological activities
[1][2][3]. Over the past fifty years, progressive improvements have been made in the exploration of new marine habitats, leading to the isolation of thousands of unique marine natural products
[2] for industrial development, such as pharmaceuticals, food ingredients, cosmetics, drug delivery systems (DDS) and industrial enzymes
[3][4][5]. Species such as algae, sponges and corals are in constant competition because they are at high risk of predation, so these species have developed chemical defense mechanisms based on the synthesis of toxic secondary metabolites
[6].
In the last decade, FDA has approved a macrolide (Halaven
®, 2010) derived from a sponge (
Halichondria okadai) for metastatic breast cancer; an antibody-drug conjugate (Adcetris
®, 2011) isolated from a sea hare (
Dolabella auricularia) for use in Hodgkin’s lymphoma and anaplastic large cell lymphoma, and an alkaloid (isolated from a tunicate) (Yondelis
®, 2015) for ovarian cancer and soft tissue sarcoma
[7][8]. Therefore, the search for antitumor compounds derived from the sea has proved to be worthy.
In the last decade, nearly fifty compounds with anti-glioma activity were isolated from anemone, brown, red and green seaweeds, invertebrates, sponges, corals, fungi and crustaceos. Most compounds were isolated from marine sponges (27%), followed by seaweeds (15%) and marine bacteria and marine corals (both with 13%). A high chemical diversity was found: anthraquinones, chromones, peptides, pyrazolidines, sesquiterpenes, tanines, saccharides, carotenoids, Bafilomycins, Actinomycins, Fradimicins, Streptoglutarimides, alkaloids and saponins. From these, sesquiterpenes (19%), alkaloids (13%) and polyketides (13%) were the most commonly described compounds with antiproliferative activity.
The majority of studies have only conducted as preliminary on drug discovery processes involving in vitro screening on glioma cell lines, including, T98G, U87MG, SHG44, U251MG, U373MG, C6 among others. None have already reached advanced stages of preclinical development with proven efficacy in vivo. However, the mechanisms of action of some compounds have been elucidated, including: (i) the decrease of levels of expression of various metabolic enzymes overexpressed in the glioma, namely glycolytic enzymes (HK2, PFKFB3, PKM2 and LDH5) by compounds 1, 2, 15 and 48 and enzymes involved in glutaminolysis (GLS) by compounds 15 and 48, and in lipogenesis (FASN) by compound 15; (ii) inhibition of the AKT pathway, from which proteins are highly expressed in tumors with a worse prognosis, by compound 7; (iii) inhibition of caspase 3/7 activity and increase of LDH activity, leading to apoptosis by compound 22; (iv) inactivation of EGFR and the PI3K-AKT signaling pathway by compounds 23–25, leading to cell death; (v) and interruption of the G2-M cell cycle and mitochondrial apoptosis by compound 40.
From the nearly fifty marine compounds, only ten (5, 27, 29, 38, 40, 41, 43–46) have potential to cross BBB, being the pyrazolidene 5, isolated from an anemone, the most potent compound (IC50 = 0.5 to 3.0 μM) followed by the plakortide 29 (IC50 = 4.0 μM) and the polyketide 38 (IC50 = 4.1 μM), both isolated from sponges and by the polyketide 40 (IC50 = 5.1 to 6.9 μM), isolated from corals. Drug delivery systems can help decreasing toxicity and providing greater BBB permeability and effectiveness within the brain. It can be expected that some of the most promising compounds derived from marine sources can be applied in the future in conjunction with DDS. On the other hand, some of the presented marine compounds may work as models for the synthesis of simplified analogs in a near future.
2. Marine Anemone
An extract prepared from the culture of a sea anemone-derived actinomycete
Streptomyces sp. ZZ406
[5] was found to have activity in inhibiting the proliferation of glioma cells and reducing the production of lactate in glioma cells
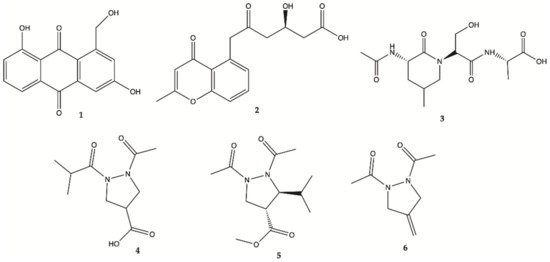
[9][10][11]. Chemical investigation of this crude active extract led to the isolation and identification of compounds
1–
4 ()
[12]. The structure of compound
1 was elucidated as 1-hydroxymethyl-8-hydroxy-anthraquinone-3-carboxylic acid, a new anthraquinone
[12]. These new compounds
1–
4 were also tested for their activity in inhibiting the proliferation of human glioma U87MG, U251 and SHG44 cells by sulforhodamine B (SRB) assay. Doxorubicin (DOX), a chemotherapeutic drug, was used as a positive control. It has been found that
1 had potent activity against different glioma cells with IC
50 values in a range of 4.7 to 8.1 μM, and good stability. Compound
2, a chromone, showed IC
50 values of 21.6–25.8 μM. Unfortunately, the new peptide
3 and new pyrazolidine derivative
4 were inactive. DOX had an antiproliferative activity with IC
50 values of 1.9–9.6 μM. Pyrazolidenes 5 and 6 were also assayed for their activity against glioma. The results showed that both pyrazolidines
5 and
6 also had anti-glioma activity with IC
50 values of 0.5 to 3.0 μM for
5 and 10.4 to 36.3 μM for
6. The cytotoxicity (CC
50) of the two active compounds
1 and
2 towards normal human astrocytes (HA) was also evaluated and an IC
50 higher than 100 μM was found showing high selectivity index (>12.3 to 21.3). They were also analyzed for their effects on the expression levels of important tumor glycolytic (regulatory) enzymes—hexokinase (HK2), 6-phosphofructo-2-kinase/2,6-bisphosphatase 3 (PFKFB3), pyruvate kinase M2 (PKM2) and lactate dehydrogenase (LDH5), highly expressed in U87MG 27 cells. Compounds
1 and
2 clearly reduced the levels of HK2, PFKFB3, PKM2 and LDH5 expression
[12].
Figure 1. Compounds
1–
6 isolated from the culture of
Streptomyces sp.
ZZ406 [12].
3. Seaweed
Seaweeds (also known as macroalgae) comprise a very large number of species of marine algae that are macroscopic and multicellular. This designation “seaweeds” includes Rhodophyta (red), Phaeophyta (brown) and Chlorophyta (green) macroalgae. Only one compound isolated from red algae was described as active in glioma cells and its mechanism of action is thought to be related to the inactivation of the AKt pathway. Regarding brown algae, at least three compounds from different chemical classes, phorotannin, saccharide and carotenoids, were described. It should also be noted that these compounds have different mechanisms of action: (i) eckol was shown to suppress PI3K-AKt activity and inhibit the Ras-Raf-1 pathway, (ii) fucoidan was reported to stimulate differentiation of osteoblasts via c-Jun N-terminal kinase (JNK), (iii) fucoxanthin showed preventive effects through different mechanisms of action. A large number of studies
[13][14][15][16] have shown that these different mechanisms of anticancer action include anti-proliferation, suppression of angiogenesis, cell cycle arrest, apoptosis induction and antidrug potential. Regarding green algae, four compounds from the same chemical class, Bafilomycins, were described with antiproliferative activity on human glioma cells
[15].
3.1. Red Seaweeds
Aplysin (
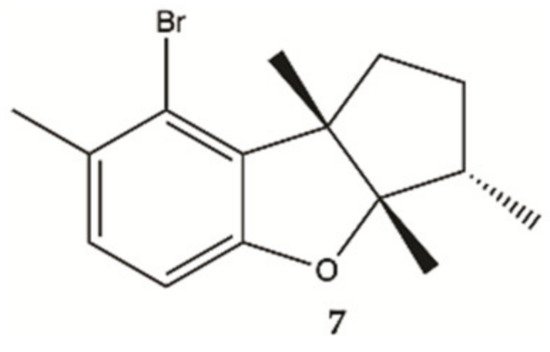
7, ), is a bromo sesquiterpene isolated from
Laurencia tristicha. This compound was found to reduce the number of invasive glioma cells U87MG and U251MG, as well as primary glioma cells, in a dose-dependent manner
[16]. Furthermore, the Akt pathway was inactivated by aplysin, and reactivation of the Akt pathway rescued its inhibitory effect on proteins associated with invasion and the invasiveness of U87MG cells. The Akt pathway is related to cell proliferation, protein synthesis, survival and motility and increased expression of these proteins has been associated with tumors with a worse prognosis. No cytotoxicity for normal cells (normal neuronal cell line, HCN2, normal liver cell line, L-02, normal endothelial cell line, HUV-EC-C and normal lung fibroblast cell line, MRC-5) was observed in the presence of aplysin (400 μg/mL)
[16].
Figure 2. Sesquiterpene
7 isolated from marine red seaweed
[16].
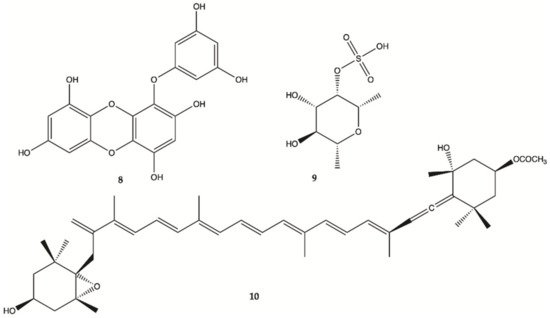
3.2. Brown Seaweeds
Eckol (
8, ), is a phlorotannin component of some brown algae, including
Ecklonia cava (Laminariaceae), and was shown to protect cells from H
2O
2-induced oxidative stress via radical quenching and catalase activation
[13]. In this study, phosphoinositide 3-kinase (PI3K)-Akt and Ras-Raf-1-Erk pathways are previously found to be activated in cancer stem-like cells whether Eckol inhibits these signaling pathways. Treatment with eckol caused a marked suppression of PI3K-Akt activities, and completely inhibited Ras-Raf-1 interaction and Raf-1 and Erk activations in sphere-forming glioma stem-like cells. It was hypothesized that eckol may enhance the sensitivity of glioma stem-like cells to anticancer treatments such as ionizing radiation or chemical drugs via inhibition of PI3K-Akt and Ras-Raf-1-Erk pathways.
Figure 3. Compounds
8–
10 isolated from brown seaweed
[13][17].
Fucoidan (
9, ), a sulfated saccharide isolated from
Fucus vesiculosus, inhibited both glioma cell-induced and monocyte-induced angiogenesis in vitro. The effects of fucoidan on T98G-induced or THP1-induced angiogenesis were also evaluated by tube formation assay using conditioned medium of fucoidan-treated cells. After 24 h, T98G and THP1 cell-induced tube formation was inhibited by fucoidan (100 mg/mL)
[17]. No apoptosis was observed in human lens epithelial cells (SRA) indicating the specific action of fucoxanthin against carcinogenic cells. Fucoxanthin (
10, ) is one of the most abundant carotenoids and contributes to more than 10% of the estimated total production of carotenoids in nature, especially in the marine environment
[18]. Fucoxanthin is a pigment that, along with chlorophylls and β-carotene, is widely distributed in brown algae and diatoms. Fucoxanthin has been described with different mechanisms of action including anti-proliferation cell cycle arrest, apoptosis induction, suppression of angiogenesis among others
[14].
3.3. Green Seaweeds
A methanol extract obtained from a culture of an actinomycete
Streptomyces sp. HZP-2216E isolated from marine green algae
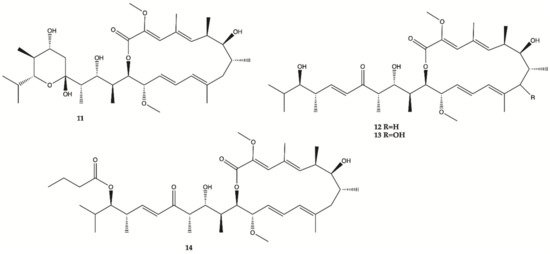
Ulva pertusa significantly inhibit proliferation of human glioma cells. Therefore, 4 Bafilomycins were isolated from this extract (
11–
14, ), and assayed for their activity against proliferation of human glioma U87-MG, U251 and SHG44 cells as well as rat glioma C6 cells by SRB assay. Bafilomycin A1 (
11), bafilomycin D (
12), 9-hydroxybafilomycin D (
13) and 23-
O-butyrylbafilomycin D (
14) showed potent activity in the suppressing of the proliferation of the four tested glioma cell lines with IC
50 values in a range from 0.35 to 2.95 μM. The control DOX had similar activity with IC
50 values of 0.48 to 1.76 μM
[15]. Unfortunately, these compounds also showed potent activity against HA with IC
50 values of 0.22 μM for
11, 0.42 μM for
12, 0.06 μM for
13, and 0.14 μM for
14.
Figure 4. Bafilomycins
11–
14 isolated from marine green algae
Ulva pertusa [15].
4. Marine Bacteria
Marine bacteria are organisms from which various compounds from different chemical families have been isolated, such as Actinomycins, Fradimicins and Streptoglutarimides. Regarding Actinomycins, three compounds were isolated, showing significant inhibition of the growth and proliferation of different glioma cell lines. Interestingly, it was discovered that Actinomycins D (15) negatively regulate various metabolic enzymes of the glioma from different metabolic pathways (glycolysis, glutaminolysis and lipogenesis). In this study, Actinomycins D (15) and V (16) showed values of IC50 of 1.01–10.06 μM and 0.42 to 1.80 μM, respectively. From the Fradimicins, two compounds were identified and isolated showing potent activity against glioma cells, but Fradimicin (18) showed an IC50 of 0.47 μM and induced apoptosis and necrosis of HCT-15, SW620, C6 cells and blocked HCT-15 cells in the phase G0/G1. Finally, from Streptoglutarimides, only two compounds were also isolated, with compound 21 showing an IC50 value of 0.05−0.22 μM and revealing more potent antiproliferative activity against glioma cells than compound 20.
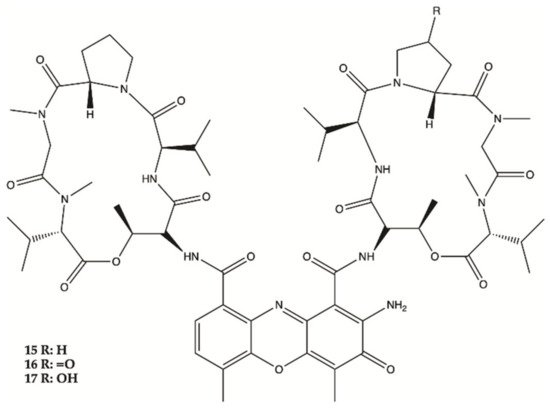
Three Actinomycins D (
15), V (
16) and X0β (
17) () produced by a
Streptomyces sp. strain, ZZ338, isolated from sea squirts, were tested for their activity against the proliferation of human glioma U251 and SHG44 cells and rat glioma C6. The data obtained from this study indicated that the referred three Actinomycins had potent activity against the proliferation of the three tested tumor cell lines, with IC
50 values from 1.01 to 10.06 nM for actinomycin D (
15), 0.42 to 1.80 nM for actinomycin V (
16), 3.26 to 25.18 nM for actinomycin X0β (
17), while the control DOX showed activity with IC
50 values in a range from 0.70 to 9.61 μM. In this study, actinomycin D (
15) was found to significantly downregulate the expression levels of several glioma metabolic enzymes, including HK2 and PKM2 from glycolysis, GLS from glutaminolysis and FASN from lipogenesis
[9].
Figure 5. Actinomycins
15–
17 isolated from sea squirts
[9].
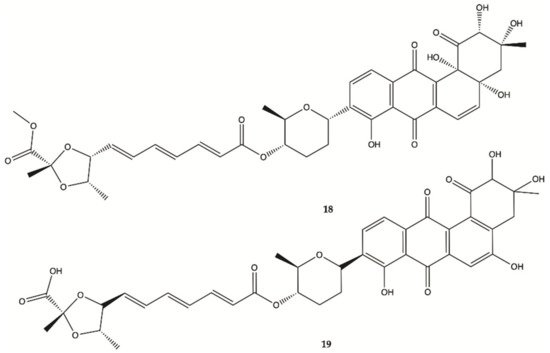
Two new Fradimicins (
18 and
19, ) were isolated and identified from marine
Streptomyces fradiae PTZ0025. Fradimicins
18 and
19 showed significantly inhibit cell growth of rat C6 glioma cells. Fradimycin
19 induced apoptosis and necrosis of C6 cells
[19].
Figure 6. Fradimicins
18 and
19 isolated from
Streptomyces fradiae PTZ0025
[19].
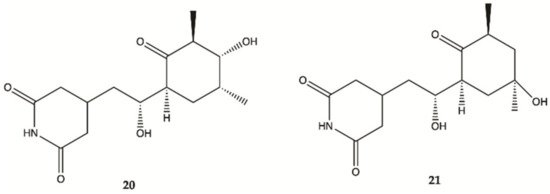
Streptoglutarimides
20 and
21 () were isolated from an actinomycete,
Streptomyces sp. ZZ741. They showed potent antiproliferative activity against human glioma U87MG and U251 cells with IC
50 values of 1.5−3.8 μM for
19 and 0.05−0.22 μM for
20 [20].
Figure 7. Streptoglutarimides
20 and
21 isolated from Actinomycete Derivates
Streptomyces sp. ZZ741
[20].
5. Marine Invertebrates
One anthraquinone and three alkaloids were isolated from marine invertebrates and studied in glioma cell lines as follows.
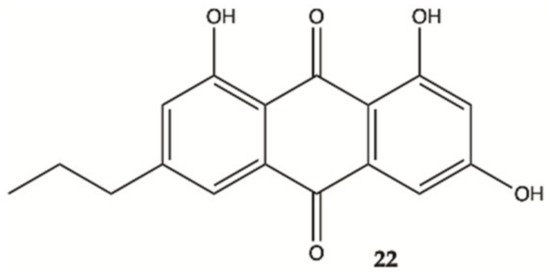
An anthraquinone (
22, ), isolated from the marine echinoderm
Comanthus sp., showed prominent toxic effects to C6 glioma cells up to 50 μM (IC
50 value of 23.2 μM). A significant increase in caspase 3/7 activity was found in C6 glioma cells showing apoptotic cell death. Incubation of C6 cells with 25 μM of compound
22 resulted in an increase in LDH activity
[21].
Figure 8. Antraquinone
22 isolated from the marine
Echinoderm Comanthus sp
[21].
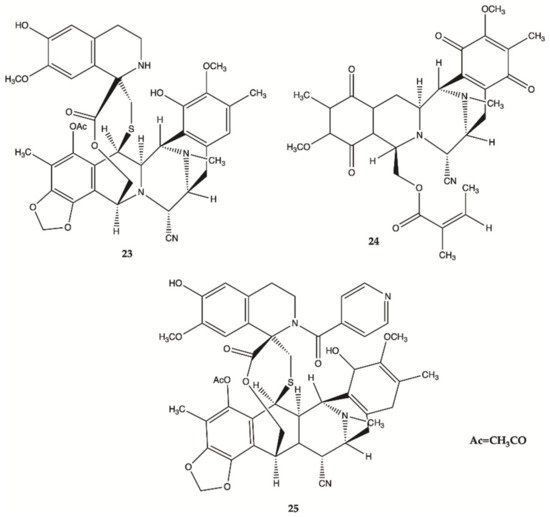
More recently, a series of 1,2,3,4-tetrahydroisoquinoline alkaloids
23–
25 were obtained from Carribean Tunicate
Ecteinascidia tubinata [22] with potent anti-cancer activities, including ecteinascidin-770 (
23, ET-770), renieramycin M (
24, RM), as well as a 2′-N-4”-pyridinecarbonyl derivative of ET-770 (
25) (). These compounds induced apoptosis of U373MG cells at nanomolar concentrations. The molecular pathways responsible for cytotoxic effects of these compounds on the human glioma cell line U373MG were characterized. The ErbB signaling pathway (EGFR) is composed of FAK/protein tyrosine kinase 2 (PTK2), Akt3, and glycogen synthase kinase 3 beta (GSK3B), serving as key molecules involved in cell movement and nervous system development. Compounds
23–
25 showed a significant relationship with the cell cycle pathway, where cell division cycle 25A (CDC25A) acts as a central molecule. Finally, we found that a specific inhibitor of GSK3B induced apoptosis of U373MG cells, supporting an anti-apoptotic role of GSK3B. These observations indicate that molecular network analysis is a useful approach not only to characterize the pathways relevant to the glioma but also to identify effective targets for network-based drugs
[22]. These alkaloids induced apoptosis of glioma cells through shared molecular mechanisms involving various pathways and targets, that play a key role in the survival and invasion of glioma cells
[22].
Figure 9. Alkaloids
23–
25 isolated from Thai marine invertebrates
[22].
6. Marine Sponges
Different chemical classes of compounds with anti-glioma activity, namely alkaloides, plakortide, sesquiterpenes and sphingosinas, were isolated from marine sponges.
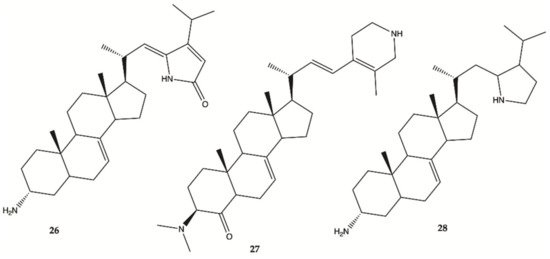
Steroidal alkaloids
26–
28 () were isolated from a sample of
Corticium sp. collected from Porth Havannah, Vanuatu, South Pacific. Cytotoxicity was evaluated in a rat glioma C6 and murine monocyte/macrophages (RAW 264) cell lines. Compound
28 to be the most active with an IC
50 value of 1.4 μg/mL against rat glioma C6, whereas compound
26 was without effect RAW 264 cell lines, but the compounds
27 (IC
50 61.0 μg/mL) and
28 (IC
50 16.2 μg/mL) were shown to be cytotoxic against RAW 264
[23].
Figure 10. Alkaloids
26–
28 isolated from Marine Sponge
Corticium sp.
[23].
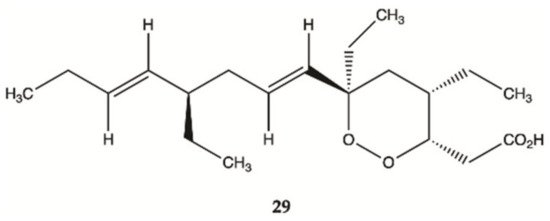
Compound
29 () isolated from sponges from the Caribbean Sea was tested for its anticancer effects on human malignant glioma cells U87MG and U373MG. Compound
29 had an IC
50 value of 4.0 μmol/L on U373MG cell line
[24].
Figure 11. Plakortide
29 isolated from Marine Sponge
Plakortis halichondroides [24].
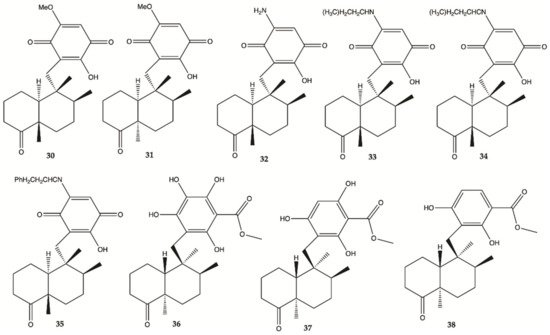
Sesquiterpene
30–
38 () were isolated from the extracts of the Hawaiian marine sponge
Dactylospongia elegans. These nine compounds showed strong to moderate cytotoxicity (IC
50 values of 2.4–19.4 μM) against the human glioma cancer cell line, U251MG
[25].
Figure 12. Sesquiterpenes
30–
38 isolated from extracts of the Hawaiian marine sponge
Dactylospongia elegans [25].
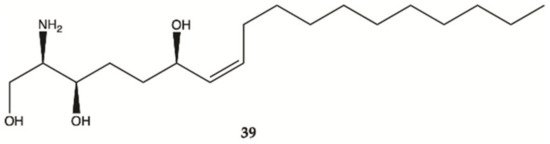
Compound
39 was isolated from a marine sponge
Haliclona tubifera, which is abundant species on the southern Brazilian coast (). This compound showed a cytotoxic effect in human glioma cell lines (U87MG), with a value lower than IC
50 < 15 μg/mL
[26].
Figure 13. Sphingosine
39 isolated from a marine sponge
Haliclona tubifera [26].
Overall, marine sponges from Brazilian, Hawaiian, Caribbean and South Pacific coasts were explored for their ability to produce secondary metabolites with anti-glioma activity. Concerning alkaloids, compound 28 was the most active. Plakortide (compound 29) has a core potent and effective against glioma cell lines. Considering sesquiterpenes, compounds 32 and 36 showed the highest potency. The only sphingosine (compound 39) isolated from marine sponges showed high activity against glioma cells.
7. Marine Corals
Seven compounds extracted from marine corals showed activity against human malignant glioma cells.
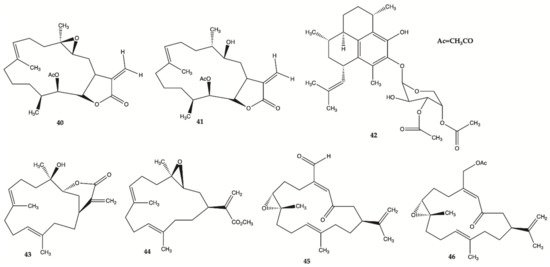
Compounds
40–
46 () are derived from specimens of Caribbean Gorgonian Octocoral
Eunicea succinea. These compounds were encoded and examined blindly for their relative cytotoxicity against human malignant glioma cells U87MG and U373MG. The cells were treated with each compound from 0 to 100 mol/L for 72 h and viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Compound
40 was stable for at least several months and was the most potent compound exhibiting IC
50 values of 5.1 mol/L for U87MG cells and 6.9 mol/L for U373MG cells. This IC
50 range is compatible with that of cisplatin, which is in the range of 5 to 10 mol/L for these types of cells. These results lead to focus on mechanism of action of compound
40 [24]. Compound
40 induced interruption of the G2-M cell cycle and apoptosis via the mitochondrial pathway. This compound was found to increase the phosphorylated JNK by >50% in both U87MG and U373MG cells. A specific JNK inhibitor, SP600125, inhibited apoptosis, confirming the involvement of the JNK pathway in cell death by compound-induced apoptosis. In addition, seven days of daily intratumor injections of compound
40 significantly suppressed the growth of malignant glioma xenografts
[24].
Figure 14. Polyketides
40–
46 isolated from
Caribbean Octocoral Eunicea succinea [24].
8. Marine Fungi
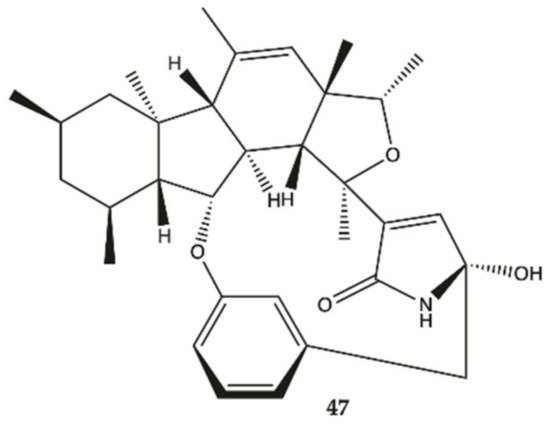
In 2018, one alkaloid with antiproliferative activity against human glioma cells was isolated from a marine fungus strain ZZ380 isolated from a wild crab
[27].
The proliferation of glioma U87MG, U251, SHG44 and C6 cells in the presence of penicipyrroether alkaloid (
47, ) was assayed by SRB assay. Compound
47 showed potent activity in inhibiting the proliferation of different glioma cells with IC
50 values of 1.64 e 5.50 μM for U87MG and U251 cells, respectively
[27].
Figure 15. Alkaloid
47 isolated from marine fungus strain ZZ380
[27].
9. Cucumbers
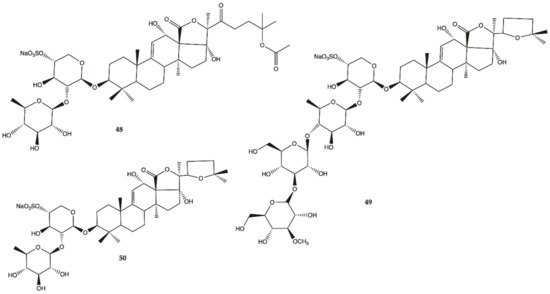
Sulfated saponins were isolated from sea cucumber
Holothuria moebii Ludwig, a species of sea cucumbers of the Holothuriidae family. For the first time, sulfated saponins
48–50 () were shown to have potent activity in suppressing the proliferation of glioma cells (C6 glioma cells, U87MG, U251 and SHG44 cells). This study also uncovered that sulfated saponin
48 could have a unique antitumor mechanism by selectively targeting multiple glioma metabolic regulators of glycolysis and glutaminolysis, hexokinase 2 (HK2), 6-phosphofructo-2-kinase/2,6-bisphosphatase 3 (PFKFB3), pyruvate kinase (PKM2) and glutaminase (GLS). It has been revealed that these metabolic regulators are related to the tumorigenesis of gliomas
[28]. Saponin
48 had no significant effect on the expression levels of HK2, PFKFB3, PKM2 and GLS in HA.
Figure 16. Sulfated saponins
48–50 isolated from
Cucumber Holothuria moebii [28].
10. Crustaceans and Fishes
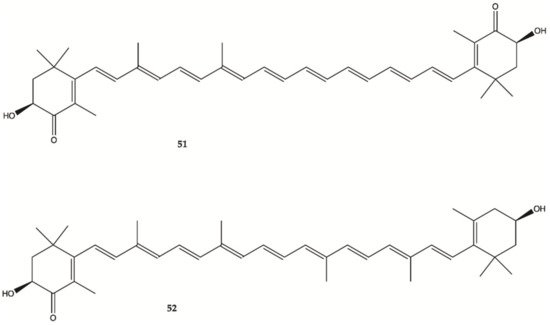
Astaxanthine (
51, ) is a red pigment found in crustaceans and fish that occurs naturally in shrimp, crab and salmon, and is also synthesized in
Haematococcus pluvialis. Adonixanthin (
52) is an intermediate product of astaxanthin ()
[29]. A cell viability assay was performed using the murine GBM cell lines GL261 and human GBM cell line U251MG cells. In GL261 cells, compounds
51 and
52 suppressed cell viability at concentration of more than 5 and 0.1 μM. In U251MG cells, compounds
51 and
52 suppressed cell viability at concentrations of more than 1 and 0.1 μM.
Figure 17. Astaxanthin (
51) Adonixanthin (
52) isolated from a red pigment
[29].
These two compounds inhibited both cell proliferation and migration in human and mouse glioma cells. Both were found to reduce the expression of phopharylated ERK1/2 and phosphorylated Akt
[29].