Blue light cystoscopy (BLC) is the most recent clinical approach in the detection and diagnosis of bladder cancer, a common type of cancer with a high rate of recurrence. Representing a significant advance over previous approaches, this photodynamic diagnostic technique uses a photosensitiser prodrug as an adjunct to white light cystoscopy to enhance the in vivo detection of malignant tissues in the bladder based on their distinctive fluorescence. Whilst it does improve detection rates, BLC remains an invasive and costly procedure. Meanwhile, a variety of noninvasive urine detection methods and related microdevices have been developed. In the following section, we provide the current context for urinary biomarker testing, including commercially available tests and recent development involving microdevices.
- noninvasive
- urinary markers
- urine
- microdevices
- bladder cancer
- biomarkers
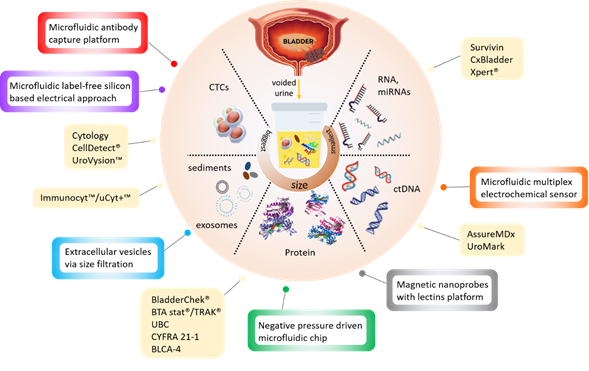
New noninvasive tests based on the detection of cancer-specific biomarkers in urine have been in development over the last decades. The different types of target biomarkers found in urine are summarised in Figure 1 below.
Figure 1. Infographic illustrating the currently available (yellow boxes) and potential microdevices (colour bordered boxes) for urinary bladder cancer diagnosis, as described in Tables 1 and 2 below.
Table 1 provides a nonexhaustive list of studies involving these biomarkers that have resulted in commercially available tests for bladder cancer diagnosis. Most efforts have focused on detecting molecular biomarkers, i.e., tumour-specific proteins such as complement factor H-related protein, nuclear matrix protein (NMP) or UBC specific glycoproteins, primarily via immunochemical methods [1]. Several urine-based tests that detect these protein biomarkers have been commercialised and six of those approved by the FDA (BTA stat, BTA TRAK, NMP22, NMP22 BladderChek, uCyt+/ImmunoCyt and UroVysion) [2][3]. Other urinary tests under development that are not, to date, recommended for diagnostic use, include UBC-Rapid/ELISA test, CYFRA 21-1 and BLCA-1/BLCA-4, which assay proteins predominantly present in metastatic cells. These urine-based assays have the advantage of being noninvasive and rapid. They also have higher sensitivity than urine cytology but tend to be less specific and many suffer from variable performance [2][3][4] (Table 1). In addition, they have a lower sensitivity than white light cystoscopy for lower grade tumours (30–60%), with specificity ranging from 60% to 90%, and false-positive results in patients with inflammatory conditions [5]. The sensitivity and specificity values reported (Table 1) are highly dependent upon the clinical setting of the studies and discrepancy, therefore, arises from differences in patient cohort (selection criteria and size, tumour grades examined) and study design (primary or recurrent tumours; initial diagnosis or surveillance). Although some of these urine-based tests have been commercialised, their sensitivities and specificities have not been sufficient to justify changes in diagnostic or surveillance protocols. So far, the application of these new urine tests tends not to improve the identification of the disease but merely increase the associated costs [6].
In the quest for an accurate urinary biomarker for bladder cancer, many new—omics biomarkers have been reported [7][8], as recently summarised in comprehensive reviews [1][9][10]. Tests targeting genomic biomarkers that are commercially available are provided in Table 1. These tests typically detect DNA methylation, mutation or mRNA expression using PCR, SAGE and/or mass spectrometry methods. The detection of next-generation “omics” biomarkers may be more accurate but has the disadvantage of relying on expensive reagents and complex analytical platforms.
Table 1. Summary of available tests based on the detection of urinary biomarkers [2][3]. A non-systematic literature research was performed using the PubMed/Medline database. Searched by using the following keywords: “bladder cancer”, “urinary markers”, “biomarkers”, “diagnosis”, “detection”, “urine biomarkers”, “NMIBC”, “surveillance”. The search was conducted in 2020.
|
Test (Manufacturer) |
Detected Biomarker |
Assay type |
Sensitivity % |
Specificity % |
Development Stage* |
FDA Approved |
Ref. |
|
Urine Cytology |
Atypical urothelial cells |
Microscopy |
33.3 |
100 |
Clinical practice |
NA |
[11] |
|
NMP22/BladderChek® (Abbott Laboratories, IL, USA) |
Nuclear mitotic apparatus proteins (Nuclear matrix protein-22) |
Sandwich ELISA/point-of-care test |
33–77 |
75–97 |
FDA approved diagnosis and follow-up |
1996/2002 |
[4] |
|
uCyt+™/Immunocyt™ (Scimedx Corporation, NJ, USA) |
Bladder tumour cell associated mucins/carcinoembryonic antigen (antibodies19A211, LDQ10 and M344) |
Immunocytochemistry |
78–90 |
77–87 |
FDA approved follow-up |
2000 |
[4] |
|
UroVysion ™ (Abbott Laboratories, IL, USA) |
Aneuploidy and loss of loci (chromosomes 3, 7, 17 and 9p21 loci) |
Multicoloured and multiprobed FISH |
50–88 |
87–98 |
FDA approved diagnosis and follow-up |
2002 |
[4] |
|
BTA stat®/TRAK® (Polymedco Inc., NY, USA) |
Complement factor H-related protein |
Dipstick immunoassay/sandwich ELISA |
61–87 |
38–87 |
FDA approved diagnosis and follow-up |
1998/1997 |
[4] |
|
UBC-Rapid/ELISA test (IDL Biotech AB, Bromma, Sweden) |
Cytoskeletal protein (cytokeratin 8 and 18) |
Sandwich ELISA/point-of-care test |
48.7–70.5 |
64.5–79.3 |
Clinical laboratory research |
|
[11] |
|
CYFRA 21-1 (Roche Diagnostics, IN, USA) |
Cytoskeletal protein (cytokeratin 19) |
Electrochemiluminescent immunoassay/ELISA/immunoradiometric assay |
82 |
80 |
Clinical laboratory research |
|
[12] |
|
BLCA-4 (Eichrom Technologies, IL, USA) |
Nuclear matrix protein (BLCA-4) |
Sandwich ELISA |
96.4 |
100 |
Clinical laboratory research |
|
[13] |
|
Survivin (Fujirebio Diagnostics Inc., PA, USA) |
Inhibitor of apoptosis gene |
Bio-dot test |
64 |
93 |
Clinical trial |
|
[14] |
|
Cx Bladder (Pacific Edge Diagnostics, PA, USA) |
mRNA expression of genes (IGF, HOXA, MDK, CDC and IL8R) |
RT-qPCR |
91 |
96 |
Clinical trial |
|
[15] |
|
AssureMDx (MDxHealth, CA, USA) |
Methylation analysis (OTX1, ONECUT2 and TWIST)/mutation analysis (FGFR3, TERT and HRAS) |
Methylation/mutation analysis |
57–83 |
59 |
Clinical laboratory research |
|
[16] |
|
Xpert® bladder cancer monitor (Cepheid Inc., CA, USA) |
mRNA expression of genes (CRH, IGF2, UPK1B, ANXA10 and ABL1) |
RT-qPCR |
73 |
77–90 |
Clinical trial |
|
[17] |
|
UroMark (Kelly:Feber Lab, UCL, UK) |
Targeted loci DNA methylation (150 CpG loci) |
Microdroplet-based PCR and NGS |
98 |
97 |
Clinical trial |
|
[18] |
|
CellDetect ® (Micromedic Technologies Ltd., Tel Aviv, Israel) |
Atypical urothelial cells |
Microscopy |
94 |
89 |
Clinical trial |
|
[19] |
|
ELISA: enzyme-linked immunosorbent assay; UBC: urinary bladder cancer; RT-qPCR: reverse transcription quantitative polymerase chain reaction; NGS: next-generation sequencing |
|||||||
* According to the ClinicalTrials.gov, a source provided by the U.S. National Library of Medicine [20]. Development stages are considered as “Clinical laboratory research” and “Clinical trial”.
In attempts to reduce the operating complexity of urinary tests without compromising their efficiency, existing (e.g., ELISA) and novel (e.g., cell membrane capacitance) detection approaches have been integrated into microdevices (Table 2). Most of these devices are still at a development stage and have not been rigorously assessed for clinical sensitivity and specificity. They target all types of bladder cancer biomarkers, including protein [21][22], DNA [23], extracellular vesicles [24][25] and whole cells [26][27][28] (Figure 1) but use advanced materials and nanotechnology to reduce analysis time and sample volumes.
Table 2. Types of microdevices for bladder cancer detection in urine.
|
Microdevices |
Detected Marker |
Assay Type |
Ref. |
|
Negative pressure-driven microfluidic chip |
APOA1 protein via antibody capture on magnetic microbead |
ELISA |
[21] |
|
Magnetic nanoprobes with lectins platform |
Glycoproteins via Glycoproteomics and CD44 expression |
Slot-blot analysis, immunohistochemistry |
[22] |
|
Microfluidic multiplex electrochemical sensor |
cfDNA via DNA hairpins bound to electrode, DNA methylation |
SPR/EIS |
[23] |
|
Microfluidic antibody capture platform |
Cancer cell capture via EpCAM on POx coating |
Point-of-care test |
[26] |
|
Antibody conjugated nanoprobes immunosensor |
Intracellular Gal-1 protein via immunosensor |
Point-of-care test |
[27] |
|
Microfluidic label-free silicon-based electrical approach |
Whole cells via membrane capacitance difference |
Flow cytometry |
[28] |
|
Microfluidic double filtration |
Extracellular vesicles via size filtration |
ELISA |
APOA1: apolipoprotein 1; SPR: surface plasmon resonance; EIS: electrochemical impedance spectroscopy; cfDNA: cell-free deoxyribonucleic acid; EVs: extracellular vesicles; EpCAM: epithelial cell adhesion molecule; Gal-1: galectin-1; POx: polyoxazoline.
For instance, a negative pressure-driven microchip integrating magnetic microbead-assisted immunocapture of bladder cancer biomarker apolipoprotein A1 (APOA1), report a measurement time of 40 min which is six times faster than a conventional ELISA test [21]. The detection of cancer-specific nucleic acid has been achieved using electrochemical impedance spectroscopy (EIS) and Surface plasmon resonance (SPR) within a microdevice containing porphyrin-tagged DNA probes [23]. Methods capable of detecting whole bladder cancer cells shed in urine are typically based on cell size, cellular features or the expression of specific proteins (e.g., intracellular galectin-1 or EpCAM). These microdevice-assisted approaches provide real-time detection in microliter volumes of urine [27] and reported specificity and sensitivity above 95% for the detection of cancer cells in spiked urine samples [26]. Microdevices provide an opportunity for the detection of novel biomarkers such as extracellular vesicles (EV) [29][30]. Tumour-derived EVs exist in various biological fluids, including urine, and carry cancer-specific proteins and nucleic acids. Technological approaches which capture and isolate bladder cancer EV through double-nanofiltration have been developed [24]. One of these approaches reported a sensitivity of 81% and a specificity of 90% in a modest cohort of 16 bladder cancer and eight healthy patients’ urine samples [25]. The advantages of these microfluidic devices over traditional EV isolation are that they require less processing steps and are, therefore, simpler and quicker (30 min). Furthermore, the final product contains nucleic acids and proteins that can be further used for genetic research which may provide personalised insight into the tumour heterogeneity. However, microdevice-based testing generally suffers from variations in the chemical and cellular composition of urine, as well as interpatient variability, more than conventional tests [31] because of the particularly low volume of the sample tested.
Overall, no urinary test based on urinary biomarker detection has yet replaced cystoscopy in screening and primary detection for NMIBC bladder cancer, according to current oncological guidelines (the American Urological Association (AUA)/Society of Urologic Oncology (SUO) [32], National Institute for Health and Care Excellence (NICE) [33], European Association of Urology (EAU) [34], and National Comprehensive Cancer Network (NCCN) [35]. Their use is not recommended for routine testing of low-risk NMIBC follow-up patients, and while they may be considered for the surveillance of high-risk NMIBC follow-up cases, the health care management plan for bladder cancer survivors still recommends including frequent cystoscopy and cytology.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics10060383
References
- Ashish Chakraborty; Shobha Dasari; Wang Long; Chandra Mohan; Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer.. null 2019, 9, 1104-1117, .
- Susanna Lintula; Kristina Hotakainen; Developing biomarkers for improved diagnosis and treatment outcome monitoring of bladder cancer. Expert Opinion on Biological Therapy 2010, 10, 1169-1180, 10.1517/14712598.2010.489546.
- Moritz Maas; Simon Walz; Viktoria Stühler; Stefan Aufderklamm; Steffen Rausch; Jens Bedke; Arnulf Stenzl; Tilman Todenhöfer; Molecular markers in disease detection and follow-up of patients with non-muscle invasive bladder cancer. Expert Review of Molecular Diagnostics 2018, 18, 443-455, 10.1080/14737159.2018.1469979.
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S.; Urinary biomarkers for diagnosis of bladder cancer: A systematic review and meta-analysis.. Annals of internal medicine 2015, 163, 922-931, .
- Peter G. Yousef; M Y Gabril; An update on the molecular pathology of urinary bladder tumors. Pathology - Research and Practice 2018, 214, 1-6, 10.1016/j.prp.2017.11.003.
- Ashish M. Kamat; Jose A. Karam; H. Barton Grossman; A. Karim Kader; Mark Munsell; Colin P. Dinney; Prospective trial to identify optimal bladder cancer surveillance protocol: reducing costs while maximizing sensitivity. BJU International 2011, 108, 1119-1123, 10.1111/j.1464-410x.2010.10026.x.
- Chong Shen; Zeyu Sun; Deying Chen; Xiaoling Su; Jing Jiang; Gonghui Li; Biaoyang Lin; Jiajun Yan; Developing Urinary Metabolomic Signatures as Early Bladder Cancer Diagnostic Markers. OMICS: A Journal of Integrative Biology 2015, 19, 1-11, 10.1089/omi.2014.0116.
- Maria Frantzi; Kim E Van Kessel; Ellen C. Zwarthoff; Mirari Marquez; Marta Rava; Núria Malats; Axel S Merseburger; Ioannis Katafigiotis; Konstantinos Stravodimos; William Mullen; et al. Development and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center study. Clinical Cancer Research 2016, 22, 4077-4086, 10.1158/1078-0432.ccr-15-2715.
- Sarah S. Dinges; Annika Hohm; Lindsey A. Vandergrift; Johannes Nowak; Piet Habbel; Igor A. Kaltashov; Leo L. Cheng; Cancer metabolomic markers in urine: evidence, techniques and recommendations. Nature Reviews Urology 2019, 16, 339-362, 10.1038/s41585-019-0185-3.
- Wei Shen Tan; Mae-Yen Tan; Pramit Khetrapal; Liqin Dong; Patricia Dewinter; Andrew Feber; John D. Kelly; Wei Phin Tan; Novel urinary biomarkers for the detection of bladder cancer: A systematic review. Cancer Treatment Reviews 2018, 69, 39-52, 10.1016/j.ctrv.2018.05.012.
- M Babjuk; M Koštı́řová; K Mudra; S Pecher; H Smolová; L Pecen; Z Ibrahim; Jan Dvoracek; L Jarolı́m; J Novák; et al. Qualitative and Quantitative Detection of Urinary Human Complement Factor H-Related Protein (BTA stat and BTA TRAK) and Fragments of Cytokeratins 8, 18 (UBC Rapid and UBC IRMA) as Markers for Transitional Cell Carcinoma of the Bladder. European Urology 2002, 41, 34-39, 10.1016/s0302-2838(01)00015-x.
- Yuan-Lan Huang; Jie Chen; Wei Yan; Ding Zang; Qin Qin; An-Mei Deng; Diagnostic accuracy of cytokeratin-19 fragment (CYFRA 21–1) for bladder cancer: a systematic review and meta-analysis. Tumor Biology 2015, 36, 3137-3145, 10.1007/s13277-015-3352-z.
- Badrinath R. Konety; Thu-Suong T. Nguyen; Gilbert Brenes; Arnold Sholder; Nancy Lewis; Sheldon Bastacky; Douglas M. Potter; Robert H. Getzenberg; CLINICAL USEFULNESS OF THE NOVEL MARKER BLCA-4 FOR THE DETECTION OF BLADDER CANCER. Journal of Urology 2000, 164, 634-639, 10.1016/s0022-5347(05)67269-2.
- Shahrokh F. Shariat; Roberto Casella; Seyed M. Khoddami; Gina Hernandez; Tullio Sulser; Thomas C. Gasser; Seth P. Lerner; Urine Detection of Survivin is a Sensitive Marker for the Noninvasive Diagnosis of Bladder Cancer. Journal of Urology 2004, 171, 626-630, 10.1097/01.ju.0000107826.78479.90.
- Yair Lotan; Paul Oʼsullivan; Jay D. Raman; Sharokh F. Shariat; Laimonis Kavalieris; Chris Frampton; Parry Guilford; Carthika Luxmanan; James Suttie; Henry Crist; et al. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma.. Urologic Oncology: Seminars and Original Investigations 2017, 35, 531.e15-531.e22, 10.1016/j.urolonc.2017.03.008.
- Willemien Beukers; Kirstin A. Van Der Keur; Raju Kandimalla; Yvonne Vergouwe; Ewout W. Steyerberg; Joost L. Boormans; Jørgen Bjerggaard Jensen; José A. Lorente; Francisco X Real; Ulrike Segersten; et al. FGFR3 , TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. Journal of Urology 2017, 197, 1410-1418, 10.1016/j.juro.2016.12.096.
- Ellen Wallace; Russell Higuchi; Malini Satya; Leena McCann; Mandy L.Y. Sin; Julia A. Bridge; Huilin Wei; Jun Zhang; Edith Wong; Andrew Hiar; et al. Development of a 90-Minute Integrated Noninvasive Urinary Assay for Bladder Cancer Detection. Journal of Urology 2018, 199, 655-662, 10.1016/j.juro.2017.09.141.
- Andrew Feber; Pawan Dhami; Liqin Dong; Patricia De Winter; Wei Shen Tan; Mónica Martínez-Fernández; Dirk S. Paul; Antony Hynes-Allen; Sheida Rezaee; Pratik Gurung; et al. UroMark—a urinary biomarker assay for the detection of bladder cancer. Clinical Epigenetics 2017, 9, 8, 10.1186/s13148-016-0303-5.
- Noa Davis; Yoram Mor; Pavel Idelevitch; Dov Terkieltaub; Vivi Ziv; Adi Elkeles; Sylvia Lew; Elimelech Okon; Menachem Laufer; Jacob Ramon; et al. A Novel Urine Cytology Stain for the Detection and Monitoring of Bladder Cancer. Journal of Urology 2014, 192, 1628-1632, 10.1016/j.juro.2014.06.079.
- ClinicalTrials.gov . NIH US National Library of Medicine. Retrieved 2020-9-25
- Yen-Heng Lin; Ying-Ju Chen; Chao-Sung Lai; Yi-Ting Chen; Chien-Lun Chen; Jau-Song Yu; Yu-Sun Chang; A negative-pressure-driven microfluidic chip for the rapid detection of a bladder cancer biomarker in urine using bead-based enzyme-linked immunosorbent assay. Biomicrofluidics 2013, 7, 024103, 10.1063/1.4794974.
- Rita Azevedo; Janine Soares; Cristiana Gaiteiro; Andreia Peixoto; Luis Lima; Dylan Ferreira; Marta Relvas-Santos; Elisabete Fernandes; Ana Tavares; Sofia Cotton; et al. Glycan affinity magnetic nanoplatforms for urinary glycobiomarkers discovery in bladder cancer. Talanta 2018, 184, 347-355, 10.1016/j.talanta.2018.03.028.
- Joanna P. Pursey; Yu Chen; Eugen Stulz; Mi Kyoung Park; Patthara Kongsuphol; Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sensors and Actuators B: Chemical 2017, 251, 34-39, 10.1016/j.snb.2017.05.006.
- Hyun-Kyung Woo; Vijaya Sunkara; Juhee Park; Tae-Hyeong Kim; Ja-Ryoung Han; Chi-Ju Kim; Hyun-Il Choi; Yoon-Keun Kim; Yoon-Kyoung Cho; Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano 2017, 11, 1360-1370, 10.1021/acsnano.6b06131.
- Li-Guo Liang; Meng-Qi Kong; Sherry Zhou; Ye-Feng Sheng; Ping Wang; Tao Yu; Fatih Inci; Winston Patrick Kuo; Lan-Juan Li; Utkan Demirci; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Scientific Reports 2017, 7, 46224, 10.1038/srep46224.
- Melanie N. MacGregor; Kym McNicholas; Kola Ostrikov; Jordan Li; Michael Michael; Jonathan Gleadle; Krasimir Vasilev; A platform for selective immuno-capture of cancer cells from urine. Biosensors and Bioelectronics 2017, 96, 373-380, 10.1016/j.bios.2017.02.011.
- Cheng-Hsin Chuang; Yi-Chun Du; Ting-Feng Wu; Cheng-Ho Chen; Da-Huei Lee; Shih-Min Chen; Ting-Chi Huang; Hsun-Pei Wu; Muhammad Shaikh; Immunosensor for the ultrasensitive and quantitative detection of bladder cancer in point of care testing. Biosensors and Bioelectronics 2016, 84, 126-132, 10.1016/j.bios.2015.12.103.
- Seied Ali Hosseini; Somayeh Zanganeh; Elaheh Akbarnejad; Fatemeh Salehi; Mohammad Abdolahad; Microfluidic device for label-free quantitation and distinction of bladder cancer cells from the blood cells using micro machined silicon based electrical approach; suitable in urinalysis assays. Journal of Pharmaceutical and Biomedical Analysis 2017, 134, 36-42, 10.1016/j.jpba.2016.11.026.
- Yu-Ru Liu; Carlos J. Ortiz-Bonilla; Yi-Fen Lee; Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond. International Journal of Molecular Sciences 2018, 19, 2822, 10.3390/ijms19092822.
- Eline Oeyen; Lucien Hoekx; Stefan De Wachter; Marcella Baldewijns; F. Ameye; Inge Mertens; Bladder Cancer Diagnosis and Follow-Up: The Current Status and Possible Role of Extracellular Vesicles. International Journal of Molecular Sciences 2019, 20, 821, 10.3390/ijms20040821.
- Adrie Van Bokhoven; M. Scott Lucia; Requisite for Collection and Distribution of Tissue and Fluid Specimens for Molecular Diagnostics and Discovery in Bladder Cancer. Molecular Pathology Library 2017, 1, 103-116, 10.1007/978-3-319-64769-2_6.
- Sam S. Chang; Stephen A. Boorjian; Roger Chou; Peter E. Clark; Siamak Daneshmand; Badrinath R. Konety; Raj Pruthi; Diane Z. Quale; Chad R. Ritch; John D. Seigne; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. Journal of Urology 2016, 196, 1021-1029, 10.1016/j.juro.2016.06.049.
- NICE; Bladder cancer: Diagnosis and management of bladder cancer.. BJU Int 2017, 120, 755-765, .
- M Babjuk; Willem Oosterlinck; Richard Sylvester; Eero Kaasinen; Andreas Böhle; Juan Palou-Redorta; EAU Guidelines on Non-Muscle-Invasive Urothelial Carcinoma of the Bladder. European Urology 2008, 54, 303-314, 10.1016/j.eururo.2008.04.051.
- NCCN Flash Updates: NCCN Guidelines® for Bladder Cancer . National comprehensive cancer network. Retrieved 2020-9-25