The biological impact of exogenous, alternating electric fields (AEFs) and direct-current electric fields has a long history of study, ranging from effects on embryonic development to influences on wound healing. Tumor treating fields (TTFields) is a form of alternating electric fields (AEF) therapy that is delivered to the tumor via electrodes placed on the skin.
- alternating electric fields (AEFs)
- bioelectrorheology
- cancer
- cell membrane
- cell modeling
- electroporation
- glioblastoma
- tumor treating fields (TTFields)
1. Introduction
Since the phenomenon’s initial discovery, the impact of exogenous electric forces on biology has prompted numerous research investigations. An iconic example is the experiment by Luigi Galvani in which electricity from lightning storms or static generators induced the twitching of the legs from frogs [1,2]. Advances in techniques to measure voltage and current gradients in biological material coupled with imaging technology to assess morphological changes in organisms have revealed that exposure to electro-magnetic occurrences can trigger a range of morphometric processes from embryological development to wound healing [2,3,4]. Frequencies for biologically relevant exposure to electromotive forces (EMFs) can range from being very low (0–300 Hz) to intermediate values (30 Hz to 400 kHz) to high and very high frequencies (1 MHz to 10 GHz, see Figure 1 [2,5]).
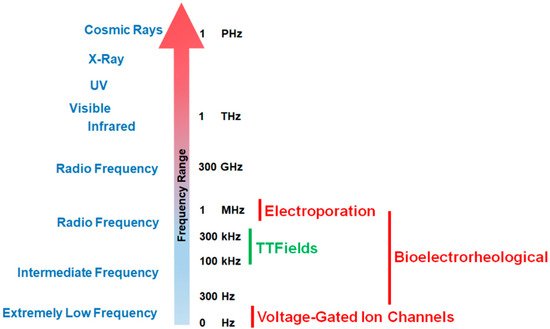
Figure 1. Effective working frequency ranges of voltage-gated calcium channels, tumor treating fields (TTFields), the bioelectrorheological model, and the electroporation model along the electromagnetic spectrum. As shown in the figure, TTFields falls within the range of intermediate frequencies while calcium channels operate at very low frequencies. By way of contrast, electroporation usually operates within the radio frequency ranges (television, radio, cell phones, microwave) while the bioelectrorheological model spans intermediate to radio frequencies.
Different EMF ranges will initiate different biological effects (Figure 1). The treatment of cancers by TTFields is a novel, validated therapy that may represent an additional paradigm (alongside surgery, radiation therapy, chemotherapy, and immunotherapy [16]) in anti-cancer treatments [17].
While not yet fully ascertained, the mechanisms of anti-cancer action by TTFields include their destabilizing effect on the tubulin dimers that have intrinsic dipole moments and which are the building blocks of microtubules, which in turn constitute the mitotic spindle [8]. By forcing microtubular filaments to align along electric field lines through the exogenous imposition of TTFields, the functionality of the mitotic spindle is interrupted in actively dividing cells [18] thereby disrupting replication [8,9,19] (Figure 2A). However, it is widely believed that AEFs in general, and TTFields in particular, display a wider range of mechanisms. In addition to destabilizing microtubules, AEFs may affect the formation of proteins involved with the mitotic spindle complex, e.g., septins [24,25].
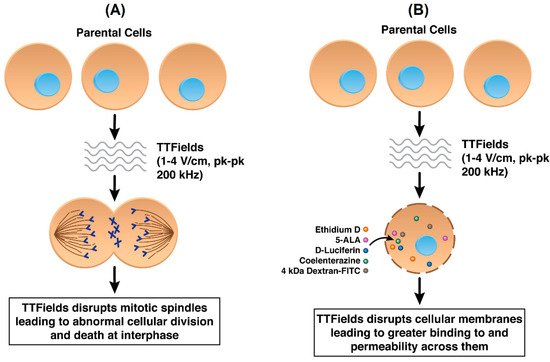
Figure 2. (A) Standard mechanism of action of TTFields in cancer cells by disrupting mitotic spindle formation (B) TTFields disrupting cancer cell plasma membranes resulting in increased permeability.
We propose three models that explain how intermediate frequency AEFs affect cellular membranes (Figure 3) and impact membrane permeability.
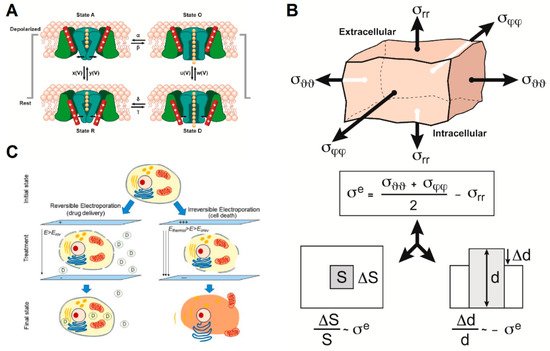
Figure 3. The three models that could partially explain the action of alternating electric fields (AEFs) on cell membrane integrity and function include: (A) Impact of AEFs on voltage-gated ion channels (adapted from [38,39]). (B) The bioelectrorheological model (adapted from [40]). (C) The electroporation model (reprinted with permission from ref. [41]. Copyright 2019 Springer Nature). Parameters are defined in the respective references and Tables S1–S3.
2. Explanatory Models
2.1. Ion Channel Activation through Effects of AEFs
The effects of alternating current (AC) electric fields on ion channels have been investigated extensively in the ranges of frequency and amplitude that affect nerve cells (neurons and nerve fibers), e.g., from direct current (DC) to 50 kHz and from 0.001 V to 1 V along the fiber, and to a lesser extent, at other frequency ranges and amplitudes [42]. Prima facie, while the amplitude of TTFields is sufficient to modulate ion channels in the cell membrane, the frequency of TTFields (100–300 kHz) would seem to preclude effects such as initiation and propagation or blocking of action potentials in nerve cells, because such effects are not seen above ~30–50 kHz [2]. Above this frequency range, the time constants of the channel gates, being 1–2 orders of magnitude greater than the periods corresponding to TTFields’ frequencies, are too long for the gates to track the amplitude modulation of the sine wave. The membrane capacitance filter becomes negligible as well, consequently resulting in the inability of a nerve fiber to initiate and propagate an action potential [42,43,44,45].
2.2. Bioelectrorheological Model
The bioelectrorheological model of the cell was proposed and has been expanded upon through a series of manuscripts by Pawlowski et al. [40,53,54,55,56,57] (Figure 3B). This model demonstrates the relationship between AEFs, shape deformations, and membrane destabilization, which can contribute to electroporation and other electric field-induced cell phenomena including electrofusion and electro-destruction.
the bioelectrorheological model addresses two forms of cell stress resulting from the application of AEFs: shear stress and extensile stress (Figure 4). Both are influenced by the electric and geometrical parameters of the system; however, they affect the cell membrane in two distinct ways. Shear stress leads to physical deformations of cell shape and form, while extensile stress reversibly destabilizes the membrane thereby leaving the cell vulnerable to electroporation, electrofusion, and electro-destruction [56]. Although the electric field strengths studied by Pawlowski and colleagues [40,53,54,55,56,57] were two orders of magnitude greater than that of TTFields, the bioelectrorheological model provides a possible explanation to the causes of electroporation by demonstrating the combined effects of heat energy and extensile stress leading to reversible cell membrane destabilization, which can directly result in electroporation. Further testing of this model on cancer cells and modifying the model to take into account contact with nearby cells and cell inhomogeneities will provide deeper insights.
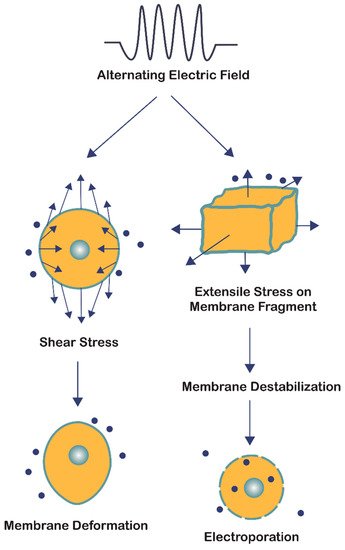
Figure 4. The bioelectrorheological model in which exogenously applied electric fields may shape deformations and destabilize membranes, which can contribute to electroporation and other electric field-induced cell phenomena including electrofusion and electro-destruction.
2.3. Electroporation Model
AEFs have been shown to permeabilize cancer cell membranes in a selective and reversible manner [27]. Electroporation is the use of high-intensity (250–3000 V/cm) DC or AC electric field pulses to cause membrane destabilization, thereby resulting in pore formation (Figure 1, Figure 3C and Figure 5) [60,61,62]. Electroporation pulses can range from nanoseconds to milliseconds in duration and tend to form aqueous pores in the plasma membrane that are on the order of ~1 nm in radius [60,62,63,64]. Whereas DC electroporation is the use of a singular pulse within a specified time interval, AC electroporation involves delivering pulses through an oscillating electric field that is characterized by its frequency (ƒ) [61].
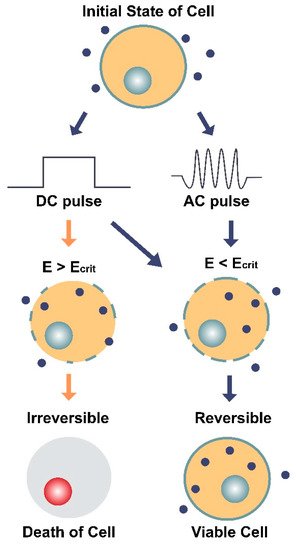
Figure 5. Illustration of the two main types of electroporation (direct current [DC] and alternating current [AC]) and their effects on a cell and its membrane. DC involves the use of short, individual pulses of electric charge whereas AC applies electric charge in an oscillating motion (increasing and decreasing) over a period of time. The dark blue circles represent molecules (average Stokes diameter ~20 nm [64]) that can only enter the cell through pores in the membrane. E is the electric field intensity (units V/cm) and Ecrit is the minimum field intensity required to reach the cell’s membrane potential threshold. The diagram shows how E can impact the cell’s survival depending on the type of current (DC or AC) and whether E is greater or less than the critical field intensity (Ecrit). When E < Ecrit, the effects of electroporation are reversible, and the cell remains viable.
Both types of electroporation are believed to cause a dielectric breakdown of the cell. However, the oscillating nature of AC electroporation is thought to provoke structural fatigue of the membrane [61], comparable to the extensile stress proposed in the bioelectrorheological model [40,53,54,55,56,57]. When both kinds of pulses (DC and AC) are applied together at their optimal settings, which involves 40 kHz frequency for the AC component, both transfection efficiency and cell survivability have been shown to increase [61].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13092283
