Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
NADPH oxidases (NOXs), mostly known as respiratory burst oxidase homologs (RBOHs), are the key producers of reactive oxygen species (ROS) in plants. A lot of literature has addressed ROS signaling in plant development regulation and stress responses as well as on the enzyme’s structure, evolution, function, regulation and associated mechanisms, manifesting the role of NOXs/RBOHs as the vital performers and center hubs during plant growth and signaling.
- NADPH oxidases (NOXs/RBOHs)
- reactive oxygen species (ROS)
- activity regulation
- plant signaling
- crop improvement
1. Introduction
Reactive oxygen species (ROS) such as superoxide anion (·O2−) and hydrogen peroxide (H2O2) are known to participate in regulation of a wide range of biological processes involved in plant development and growth, as well as adaptation to biotic and abiotic stresses [1][2][3][4][5]. NADPH oxidases (NOXs), also well known as respiratory burst oxidase homologs (RBOHs), are key enzymes for ROS production in plants. As the molecular “hubs” during ROS-mediated signal transduction pathways, NOXs/RBOHs in plants have recently received considerable attention, and more and more NOX/RBOH gene homologs have been identified in a variety of plant species [6][7][8]. The functions and regulatory mechanisms of the NOXs/RBOHs in plants have been successively reported. Recently, we identified 46 NOX family genes in wheat genome, and a systematic analysis in their transcriptional expressions reveals their vital but diverse roles in plant growth regulation and stress responses [8]. To date, more than 150 protein members of the NOXs/RBOHs family have been identified and/or characterized in various plant species (Supplementary Table S1, could be found in https://www.mdpi.com/2073-4409/9/2/437#supplementary). and among them, at least 56 members have been fully elucidated in functions. These NOXs are expressed in whole plants or specific organs/tissues relying on development stage and are functioning in many developmental processes and stress responses (Supplementary Table S1). In addition, recent advances in plant cell growth, hormone interaction and calcium signaling, as well as biotic and abiotic stress responses, have shown that NOXs/RBOHs may act as the center hubs of multiple signaling pathways of plants. Many important signaling systems functioning in plant immunity and/or hormonal and abiotic stress responses, including, OsCERK1/OsCEBiP(OsLyp4/6)-OsRLCK176/185, FLS2(EFR)/BAK1-BIK1, CERK1/LYK5(LYM1/3)-BIK1, FER context and hormonal signaling stuffs, are all directly or indirectly associated with NOXs/RBOHs activation regulation.
2. Protein Structure and Evolution of Plant NOXs/RBOHs
2.1. Highly Conservative Structure
NADPH oxidases (NOXs) are key enzymes of ROS generation and thus play crucial roles in a variety of biological processes in different kingdoms of life [9][10][11]. Since the first NADPH oxidase was identified in human phagocytic cells, more and more NOXs/RBOHs were identified in many other species including animals, higher plants and fungi [5][7][8]. In fungi, there are four types of NOXs assigned as NOXA, NOXB, NOXC and FRE [12]. In animals, there are seven types of NOXs, named as NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2 [11]. However, in plants, only NOX5-like-type NOXs were found except the ancestral NOXs, ferric reduction oxidases (FROs), which are considered as the isoforms of yeast ferric-chelate reductases (FREs) [6][7][11]. All NOX family members are transmembrane proteins that transport electrons across biological membranes to reduce oxygen to superoxide anion (·O2−) [10]. In accordance with this preserved function, there are conserved structural properties of NOX enzymes that are common to all family members.
The earliest and most comprehensive studies about NOXs were largely carried out in phagocytic cells, in which their functions involve the destruction of pathogenic microorganisms by producing ROS [13]. The phagocytic cell, NADPH oxidase, is a protein complex that is comprised of several protein components, which can be divided into two groups. The first is a membrane-bound NADPH-binding flavocytochrome b558, which is comprised of a glycosylated transmembrane protein gp91phox (β subunit) and a non-glycosylated p22phox subunit (α subunit). The gp91phox contains the entire electron transport chain from NADPH to molecular oxygen to produce ·O2− outside the plasma membrane [14][15]. The second group comprises four regulatory proteins: p47phox, p67phox and p40phox phosphoproteins and Rac1 or Rac2 (small GTP (Guanosine triphosphate)-binding protein). These four regulatory proteins could be translocated to the plasma membrane and form an enzyme complex with the first group after being activated. In this process, Rac1 or Rac2 is independently activated and translocated to assemble with the oxidase effector system, each of them endows the enzyme with guanine nucleotide sensitivity as it interacts with the activator component, p67phox, in a GTP-dependent manner. The p47phox component acts as a critical phosphorylation-dependent adaptor molecule that enables the interactions between p67phox and the flavocytochrome [16]. Further studies have shown that NADPH oxidase is also present in many other types of mammalian cells where the level of ROS generated by it is much lower and the processes of ROS generation last much longer. In this case, ROS can be used as regulatory and signal molecules for modulation of metabolic processes, leading to various biological effects [17].
Although NOXs have many isoforms (homologs), the full spectrum is only found in the animal kingdoms. A mammal genome generally contains seven genes encoding gp91phox homologs: five close relatives of gp91phox homologs (Nox1–Nox5), and two distant relatives of gp91phox homologs (Duox1 and Duox2) [10][18]. All of them share FAD (flavin adenine dinucleotide) - and NADPH-binding sites in the C-terminal domain, two heme binding sites, a functional oxidase domain responsible for ·O2− production, and some degree of sequence similarity. However, Nox5, Duox1 and Duox2 are different kinds relative to Nox1-4: Nox5 possesses four sites (motifs) of the EF hand type (elongation factor), while both Duox1 and Duox2 possess two sites of the EF hand type, which are characteristic of specific calcium-binding domains [17]. Moreover, except for Duox1 and Duox2 which possess an extra transmembrane domain with peroxidase activity, all of these homologs contain six transmembrane domains.
In stark contrast to the multienzyme complex of NADPH oxidase in mammals, plants only possess NOX5-like NOXs, which are also called respiratory burst oxidase homologs (RBOHs), even though multiple members exist in different species [6][11][19]. The typical NOXs all possess four conserved domains, namely NADPH_Ox (Pfam accession number PF08414), Ferric_reduct (PF01794), FAD_binding_8 (PF08022) and NAD_binding_6 domain (PF08022). At the same time, the distribution of amino acid residues in every domain is quite similar but not identical among the NOX/RBOH members [8]. However, further studies indicate some exceptions. The cytoplasm of tomato suspension culture cells contains protein components similar to animal p67phox and p40phox, which are activated by a fungal elicitor and can move to the plasmalemma and incorporate into the membrane cytoskeleton [17][20]. Furthermore, our recent research showed that some members have one more NADPH_Ox domain, such as TaNOX3 and TaNOX6 possessing two NADPH_Ox domains in wheat [8]. Besides the typical NOXs, some ferric reduction oxidases (FROs), which are considered as the isoforms of yeast FREs, were also found in high plants [6]. It was identified that FROs are closely related to typical plant NOXs but differences still exist: FROs contain six membrane-spanning domains, two hemes and conserved motifs involved in NADPH and FAD binding but lack NADPH_Ox domain and several calcium-binding EF-hand motifs that the typical NOX proteins possess [10][19][21]. Moreover, another new type of NOXs was put forward and assigned as NOX-likes, which have an NADPH_Ox domain but lack one or two other domains that typical NOXs possess [8].
2.2. Complex Evolution History
The typical NOX family, being identified only in terrestrial plants, underwent a complex evolution. According to our previous study, NOXs and FROs in plants can be divided into four well-conserved groups represented as NOX, FRO I, FRO II and FRO III, and an evolution model was constructed [7]. In this model, all FRO family members originated from a common ancestor which contains only the Ferric_reduct domain. During the evolutionary process, this ancestor obtained FAD_binding_8 and NAD_binding_6 domains first by gene fusion and duplication, and then clustered into FRO I, FRO II and FRO III subfamilies. FRO III mainly exists in fungi and FRO I, perceived as ancient NOX, mainly exists in animals and in two kinds of algae (rhodophytes and chlorophytes). After that, FRO I obtained another important domain-NADPH_Ox and converted into the typical NOXs in plants. FRO IIs exist both in chlorophytes and land plants but NOXs only exist in land plants. Clearly, the gene constructions become more and more complicated from FROs to NOXs over the course of evolution. In addition to domain gain and gene duplication, gene fusion as well as exon shuffling might also be involved in the evolution for biological diversity and functional divergence of the NOX gene family [7][22][23][24][25]. More recently, we found that TaFROs encoded by the genes located on Chr 1 are much closer to the typical TaNOXs in phylogenetics than those on Chr 2, and the TaFROs on Chr 2 might be more ancient forms of TaNOXs in wheat [8].
3. NOXs/RBOHs in Plant Development
The wide existence of NOXs/RBOHs in plants in some way indicates their great importance. Experiments from recent studies did show that NOXs/RBOHs play important and diverse roles in many areas including development regulation as well as the response to biotic and abiotic stresses and hormone signaling (Figure 1).
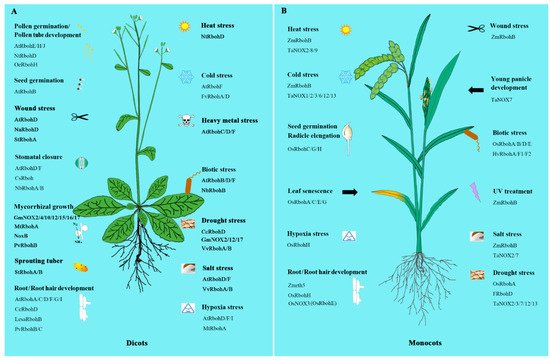
Figure 1. The involvement of NADPH oxidases/respiratory burst oxidase homologs (NOXs/RBOHs) in dicots and monocots. A. The NOXs/RBOHs in dicots. B. The NOXs/RBOHs in monocots. All the acronyms of Rbohs, NOXs, NoxB and rth5 represent the NOX/RBOH homologues from various species. Just as, AtRbohs represent the NOX/RBOH homologues from Arabidopsis thaliana, CsRbohs from Cucumis sativus, FRbohs from Festuca arundinacea, FvRbohs from Fragaria x ananassa, GmRbohs from Glycine max, HvRbohs from Hordeum vulgare, LesaRbohs from Lepidium sativum, MtRbohs from Medicago truncatula, NaRbohs from Nicotiana attenuate, NbRbohs from Nicotiana benthamiana, NoxB from Fusarium oxysporum, NtRbohs from Nicotiana tabacum, OeRbohs from Olea europaea L, OsRbohs from Oryza sativa L, PvRbohs from Phaseolus vulgaris, StRbohs from Solanum tuberosum, TaNOXs from Triticum aestivum, VvRbohs from Vitis vinifera, ZmRbohs and Zmrth5 from Zea mays. Please refer to Supplementary Table S1 for the detailed information of NOXs/RBOHs in the supplementary data.
3.1. Pollen Germination and Pollen Tube Growth
NOX enzyme, by producing ROS, is needed to sustain the normal rate of pollen tube growth and this is likely to be a general mechanism in the control of tip growth of polarized plant cells [26][27]. NOXs generate tip-localized, pulsating amounts of H2O2 that functions, possibly through Ca2+ channel activation, to maintain a steady, tip-focused Ca2+ gradient in pollen tube tip during growth [28]. On the other hand, pollen NOX can be activated by Ca2+ and Ca2+ can increase its activity in vivo [26][29]. This process would happen especially when the pollen grains are hydrated under mild conditions, so the activity of pollen NOX could be concentrated in those insoluble fractions, which could facilitate the exposure of tissues to ROS produced by this enzyme. It is worth mentioning that the extent of this exposure could differ among the plant families according to where the NOX resides in pollen grains and during hydration in the mucosa [30]. In addition, pollen NOX can also be activated by low abundant signaling phospholipids, such as phosphatidic acid (PA) and phosphatidylinositol 4, 5-bisphosphate in vitro and in vivo, so there is a possible synergism between Ca2+ and phospholipid-mediated NOX activation in pollen [29]. In plants, ROP/RAC GTPases (the subfamily of Rho-type GTPases, which belong to the Rat sarcoma superfamily of small GTP-binding proteins) are also necessary for normal pollen tube growth by regulating ROS production [29]. Besides these items mentioned above, O3 was also indicated to increase ragweed pollen allergenicity through stimulation of ROS-generating NADPH oxidase [31]. Recently, two NOXs/RBOHs, AtRbohH and AtRbohJ, have been shown to localize at the plasma membrane of pollen tube tip and the ROS production by the NOXs/RBOHs presumably plays a critical role in the positive feedback regulation that maintains the pollen tube tip growth [32][33]. Furthermore, apoplastic ROS derived from AtRbohH and AtRbohJ are involved in pollen tube elongation by affecting the cell wall metabolism [33]. More intriguing is that ectopic expressing of AtRbohC/hair root2 (hrd2) in pollen tubes could restore atrbohH and atrbohJ defects in tip growth of pollen tubes [34], which implies that AtRbohC/hrd2 also plays roles in regulating the development of pollen tubes. Moreover, AtRbohE was also suggested to be critical for programmed cell death (PCD) and pollen development in Arabidopsis thaliana L. [35]. Meanwhile, OeRbohH, possessing a high degree of identity with AtRbohH/J, plays an important role in pollen germination and pollen tube growth in olive [36]. Genetic interference with the temporal ROS pattern by manipulating NOX/RBOH genes, affected the timing of tapetal PCD (programmed cell death) and resulted in aborted male gametophytes [35]. All in all, we still cannot figure out how many factors are related to the regulation of pollen NOX. Nevertheless, what we already know is that pollen NOXs play a significant part in the regulation of pollen germination.
3.2. Root and Root Hair Development
The elongation of roots and root hairs is essential for uptake of minerals and water from the soil. Ca2+ influx from the extracellular store is required for cell elongation in roots [37]. It was suggested that plasma membrane NOXs/RBOHs and H+-ATPases (a H+ pump by coupling with energy of ATP hydrolysis on plasma membranes) are functionally synchronized and they work cooperatively to maintain the membrane electrical balance while mediating plant cell growth through wall relaxation [38]. Observations on maize roots indicate that the activities of plasma membrane-associated NADPH oxidase respond both to gravity and to imposed centrifugal forces [39]. In an early study, AtRHD2, a NADPH oxidase in Arabidopsis, was reported as controlling root development by making ROS that regulates plant cell expansion through the activation of Ca2+ channels [40]. Further, both AtRbohC/RHD2 and ROP (RHO of plants) GTPases were found to be required for normal root hair growth by regulating ROS production [41]. Coincidentally, the maize (Zea mays L.) roothairless5 (rth5) which encodes a monocot-specific NADPH oxidase, was found to be responsible for establishing the high levels of ROS in the tips of growing root hairs [42]. In rice, OsNOX3 was also reported to play critical roles in root hair initiation and elongation by regulating the content of superoxide and hydrogen peroxide in root hair tips [27]. In addition, both AtRbohD and AtRbohF are essential for ABA (abscisic acid)-promoted ROS production in Arabidopsis roots, and ROS subsequently activate Ca2+ signaling as well as reduce auxin sensitivity of roots, thus positively regulating ABA-inhibited primary root growth [43]. Moreover, AtRbohD and AtRbohF negatively modulate lateral root development by changing the peroxidase activity and increasing the local generation of ·O2- in primary roots in an auxin-independent manner [44]. Similar results were also acquired in the legume-rhizobia symbiosis and legumes use different RBOHs for different stages of nodulation [45][46]. Moreover, nitric oxide (NO) can activate NADPH oxidase activity, resulting in increased generation of ·O2-, which subsequently induces growth of adventitious roots and acts downstream of auxin action in the process of root growth and development [47]. These results suggest a vital role of NOXs/RBOHs in root and root hair development in plants.
3.3. Seed Germination
NOXs/RBOHs also play important roles in seed germination. The functional mechanism has been proposed in many plant species, such as Arabidopsis thaliana L. rice (Oryza sativa L.) and barley (Hordeum vulgare L.). AtRbohB is a major producer of ·O2- in germinating seeds, and inhibition of the ·O2- production by diphenylene iodonium (DPI) leads to a delay in seed germination of Arabidopsis and cress [48]. In rice, OsNOX5, 7 and 9 might play crucial roles in radicle and root elongation during seed germination by regulating ROS production [49]. Similarly, ·O2- produced by NADPH oxidase also regulates seed germination and seedling growth in barley [50][51]. Moreover, NOX/RBOH-mediated ROS production promotes gibberellic acid (GA) biosynthesis in barley embryos through regulation of HvKAO1 and HvGA3ox1 proteins, while GA induces and activates NOXs/RBOHs for ROS production in aleurone cells to induce α-amylase activity of the cells and therefore increases seed germination [5][52][53].
3.4. Plant-Microorganism Ineractions
Phaseolus vulgaris NADPH oxidase is crucial for successful rhizobial colonization and probably maintains proper infection thread growth and shape [54]. Moreover, it also has critical roles in reducing arbuscular mycorrhizal fungal (AMF) colonization. Overexpression of PvRbohB augments nodule efficiency by enhancing nitrogen fixation and delaying nodule senescence but impairs AMF colonization [55]. A Medicago truncatula NADPH oxidase, MtRbohA, has similar effects. It is significantly upregulated in Sinorhizobium meliloti-induced symbiotic nodules, while hypoxia prevailing in the nodule-fixing zone may stimulate MtRbohA expression, which would, in turn, lead to the regulation of nodule functioning [56]. Moreover, NoxA, a NADPH oxidase isoform in the grass endosymbiont Epichloë festucae, was identified as essential for the establishment of systemic compatible infections in host plants [50]. ROS produced by NoxA or NoxB (from Fusarium oxysporum) regulate hyphal growth of a fungal pathogen towards roots of the host plants to maintain a mutualistic and symbiotic interaction [57][58]. Recently, four GmNOXs from soybean genome (Glycine max) also showed strong expression in nodules, pointing to their probable involvement in nodulation [59]. All these results suggest that, NOXs/RBOHs, regulated by several different factors, play a significant role in mutualistic and symbiotic processes between plant and microorganism.
3.5. Fungal Development
When it comes to fungi, NOXs/RBOHs participate in a wide range of biological processes from their growth, differentiation and reproduction, to rhizobial colonization. In Aspergillus nidulans, NoxA plays crucial roles in fungal physiology and differentiation by generating ROS [60]. Moreover, genetic analysis of Δnox2 (lacking the NADPH oxidase 2 gene), Δnox1 (lacking the NADPH oxidase 1 gene) and a transcription factor deletion mutant Δste12 in Sordaria macrospora, reveals that the mutation of NOXs could lead to ascospore germination defect [61]. In yeast, NoxA, NoxB and their associated regulators (BemA, Cdc24 and NoxR) have distinct or overlapping functions for the regulation of different hyphal morphogenesis processes [57]. Furthermore, in Neurospora crassa, NOX-1 elimination results in complete female sterility, decreased asexual development and reduced hyphal growth; whereas, a lack of NOX-2 does not affect any of these processes but led to the production of sexual spores that failed to germinate [62]. In this study, the function of NOX-generated ROS acting as critical cell differentiation signals highlights a novel role for ROS in the regulation of fungal growth [62]. The function of NOX-generated ROS in regulating the reproductive process was also found in other fungi. In Botrytis cinerea, NOX complexes are essential for conidial anastomosis tubes’ formation and fusion [63].
3.6. Other Aspects
Besides functioning in the regulation of pollen germination, root development and seed germination, as well as fungal development, NOXs/RBOHs have other effects as well. It was suggested that solar ultraviolet irradiation regulates anthocyanin synthesis in apple peel by modulating the production of ROS via NADPH oxidase [64]. Moreover, chloroplastic NADPH oxidase-like activity mediates perpetual H2O2 generation, which probably induces apoptotic-like cell death of B. napus leaf protoplasts [65]. Furthermore, it was reported that among the seven homologues of NADPH oxidases in potato, the expression of StRbohA and StRbohB was detected in particular when dormancy break [66]. These results suggest very extensive roles of NOXs/RBOHs in the plant kingdom, participating in various important biological processes.
This entry is adapted from the peer-reviewed paper 10.3390/cells9020437
References
- Gapper, C.; Dolan, L. Control of Plant Development by Reactive Oxygen Species. Plant Physiol. 2006, 141, 341–345.
- Torres, M.A. Ros in Biotic Interactions. Physiol. Plant. 2010, 138, 414–429.
- Mittler, R.V.; Suzuki, S.; Miller, N.; Tognetti, G.; Vandepoele, V.B.; Gollery, K.; Shulaev, M.; Van Breusegem, V.F. Ros Signaling: The New Wave? Trends Plant Sci. 2011, 16, 300–309.
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A Burst of Plant NADPH Oxidases. Trends Plant Sci. 2012, 17, 9–15.
- Kaur, G.; Sharma, A.; Guruprasad, K.; Pati, P.K. Versatile Roles of Plant NADPH Oxidases and Emerging Concepts. Biotechnol. Adv. 2014, 32, 551–563.
- Sagi, M.; Fluhr, R. Production of Reactive Oxygen Species by Plant NADPH Oxidases. Plant Physiol. 2006, 141, 336–340.
- Chang, Y.L.; Li, W.Y.; Miao, H.; Yang, S.Q.; Li, R.; Wang, X.; Li, W.Q.; Chen, K.M. Comprehensive Genomic Analysis and Expression Profiling of the Nox Gene Families under Abiotic Stresses and Hormones in Plants. Genome Biol. Evol. 2016, 8, 791–810.
- Hu, C.H.; Wei, X.Y.; Yuan, B.; Yao, L.B.; Ma, T.T.; Zhang, P.P.; Wang, X.; Wang, P.Q.; Liu, W.; Tai, L.; et al. Genome-Wide Identification and Functional Analysis of NADPH Oxidase Family Genes in Wheat during Development and Environmental Stress Responses. Front. Plant Sci. 2018, 9, 906.
- Torres, M.A.; Dangl, J.L. Functions of the Respiratory Burst Oxidase in Biotic Interactions, Abiotic Stress and Development. Curr. Opin. Plant Biol. 2005, 8, 397–403.
- Bedard, K.; Krause, K.H. The Nox Family of Ros-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313.
- Bedard, K.; Lardy, B.; Krause, K.H. Nox Family NADPH Oxidases: Not Just in Mammals. Biochimie 2007, 89, 1107–1112.
- Aguirre, J.; Rios-Momberg, M.; Hewitt, D.; Hansberg, W. Reactive Oxygen Species and Development in Microbial Eukaryotes. Trends Microbiol. 2005, 13, 111–118.
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The Neutrophil NADPH Oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344.
- Lambeth, J.D. Nox Enzymes and the Biology of Reactive Oxygen. Nat. Rev. Immunol. 2004, 4, 181–189.
- Cross, A.R.; Segal, A.W. The NADPH Oxidase of Professional Phagocytes—Prototype of the Nox Electron Transport Chain Systems. BBA Bioenerg. 2004, 1657, 1–22.
- Leto, T.L.; Geiszt, M. Role of Nox Family NADPH Oxidases in Host Defense. Antioxid. Redox Sign. 2006, 8, 1549–1561.
- Glyan’ko, A.K.; Ischenko, A.A. Structural and Functional Characteristics of Plant NADPH Oxidase: A Review. Appl. Biochem. Microbiol. 2010, 46, 463–471.
- Rada, B.; Leto, T. Oxidative Innate Immune Defenses by Nox/Duox Family NADPH Oxidases. In Contributions to Microbiology; Egesten, A., Schmidt, A., Herwald, H., Eds.; Karger: Basel, Switzerland, 2008; pp. 164–187.
- Wang, G.F.; Li, W.Q.; Li, W.Y.; Wu, L.C.; Zhou, Y.; Chen, K.M. Characterization of Rice NADPH Oxidase Genes and Their Expression under Various Environmental Conditions. Int. J. Mol. Sci. 2013, 14, 9440–9458.
- Xing, T.; Higgins, V.J.; Blumwald, E. Race-Specific Elicitors of Cladosporium Fulvum Promote Translocation of Cytosolic Components of NADPH Oxidase to the Plasma Membrane of Tomato Cells. Plant Cell 1997, 9, 249–259.
- Wang, X.; Zhang, M.M.; Wang, Y.J.; Gao, Y.T.; Li, R.; Wang, G.F.; Li, W.Q.; Liu, W.T.; Chen, K.M. The Plasma Membrane NADPH Oxidase Osrboha Plays a Crucial Role in Developmental Regulation and Drought-Stress Response in Rice. Physiol. Plant. 2016, 156, 421–443.
- Morgante, M.; Brunner, S.; Pea, G.; Fengler, K.; Zuccolo, A.; Rafalski, A. Gene Duplication and Exon Shuffling by Helitron-Like Transposons Generate Intraspecies Diversity in Maize. Nat. Genet. 2005, 37, 997–1002.
- Van de Peer, Y.; Fawcett, J.A.; Proost, S.; Sterck, L.; Vandepoele, K. The Flowering World: A Tale of Duplications. Trends Plant Sci. 2009, 14, 680–688.
- Kaessmann, H. Origins, Evolution, and Phenotypic Impact of New Genes. Genome Res. 2010, 20, 1313–1326.
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene Duplication as a Major Force in Evolution. J. Genet. 2013, 92, 155–161.
- Potocky, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Zarsky, V. Reactive Oxygen Species Produced by NADPH Oxidase Are Involved in Pollen Tube Growth. New Phytol. 2007, 174, 742–751.
- Wang, S.S.; Zhu, X.N.; Lin, J.X.; Zheng, W.J.; Zhang, B.T.; Zhou, J.Q.; Ni, J.; Pan, Z.C.; Zhu, S.H.; Ding, W.N. OsNOX3, encoding a NADPH oxidase, regulates root hair initiation and elongation in rice. Biol. Plant. 2018, 62, 732–740.
- Boisson-Dernier, A.; Lituiev, D.S.; Nestorova, A.; Franck, C.M.; Thirugnanarajah, S.; Grossniklaus, U. Anxur Receptor-Like Kinases Coordinate Cell Wall Integrity with Growth at the Pollen Tube Tip via NADPH Oxidases. PLoS Biol. 2013, 11, e1001719.
- Potocky, M.; Pejchar, P.; Gutkowska, M.; Jimenez-Quesada, M.J.; Potocka, A.; Alche Jde, D.; Kost, B.; Zarsky, V. NADPH Oxidase Activity in Pollen Tubes Is Affected by Calcium Ions, Signaling Phospholipids and Rac/Rop Gtpases. J. Plant Physiol. 2012, 169, 1654–1663.
- Wang, X.L.; Takai, T.; Kamijo, S.; Gunawan, H.; Ogawa, H.; Okumura, K. NADPH Oxidase Activity in Allergenic Pollen Grains of Different Plant Species. Biochem. Biophys. Res. Commun. 2009, 387, 430–434.
- Pasqualini, S.; Tedeschini, E.; Frenguelli, G.; Wopfner, N.; Ferreira, F.; D’Amato, G.; Ederli, L. Ozone Affects Pollen viability and Nad(P)H Oxidase Release from Ambrosia Artemisiifolia Pollen. Environ. Pollut. 2011, 159, 2823–2830.
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-Activated Reactive Oxygen Species Production by Arabidopsis Rbohh and Rbohj Is Essential for Proper Pollen Tube Tip Growth. Plant Cell 2014, 26, 1069–1080.
- Kaya, H.; Iwano, M.; Takeda, S.; Kanaoka, M.M.; Kimura, S.; Abe, M.; Kuchitsu, K. Apoplastic Ros Production Upon Pollination by Rbohh and Rbohj in Arabidopsis. Plant Signal. Behav. 2015, 10, e989050.
- Kaya, H.; Takeda, S.; Kobayashi, M.J.; Kimura, S.; Iizuka, A.; Imai, A.; Hishinuma, H.; Kawarazaki, T.; Mori, K.; Yamamoto, Y.; et al. Comparative Analysis of the Reactive Oxygen Species-Producing Enzymatic Activity of Arabidopsis NADPH Oxidases. Plant J. 2019, 98, 291–300.
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal Production of Reactive Oxygen Species by NADPH Oxidase Is Critical for Tapetal Programmed Cell Death and Pollen Development in Arabidopsis. Plant Cell 2014, 26, 2007–2023.
- Jimenez-Quesada, M.J.; Traverso, J.A.; Potocky, M.; Zarsky, V.; Alche, J.D. Generation of Superoxide by Oerbohh, a NADPH Oxidase Activity During Olive (Olea Europaea L.) Pollen Development and Germination. Front. Plant Sci. 2019, 10, 1149.
- Cramer, G.R.; Jones, R.L. Osmotic Stress and Abscisic Acid Reduce Cytosolic Calcium Activities in Roots of Arabidopsis Thaliana. Plant Cell Environ. 1996, 19, 1291–1298.
- Majumdar, A.; Kar, R.K. Congruence between Pm H+-Atpase and NADPH Oxidase During Root Growth: A Necessary Probability. Protoplasma 2019, 255, 1129–1137.
- Bacon, E.; Morre, D.J. Plasma Membrane Nadh Oxidase of Maize Roots Responds to Gravity and Imposed Centrifugal Forces. Plant Physiol. Biochem. 2001, 39, 487–494.
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D. Reactive Oxygen Species Produced by NADPH Oxidase Regulate Plant Cell Growth. Nature 2003, 422, 442–446.
- Jones, M.A.; Raymond, M.J.; Yang, Z.; Smirnoff, N. NADPH Oxidase-Dependent Reactive Oxygen Species Formation Required for Root Hair Growth Depends on Rop Gtpase. J. Exp. Bot. 2007, 58, 1261–1270.
- Nestler, J.; Liu, S.Z.; Wen, T.J.; Paschold, A.; Marcon, C.; Tang, H.M.; Li, D.L.; Li, L.; Meeley, R.B.; Sakai, H.; et al. Roothairless5, Which Functions in Maize (Zea Mays L.) Root Hair Initiation and Elongation Encodes a Monocot-Specific NADPH Oxidase. Plant J. 2014, 79, 729–740.
- Jiao, Y.; Sun, L.; Song, Y.; Wang, L.; Liu, L.; Zhang, L.; Liu, B.; Li, N.; Miao, C.; Hao, F. Atrbohd and Atrbohf Positively Regulate Abscisic Acid-Inhibited Primary Root Growth by Affecting Ca2+ Signalling and Auxin Response of Roots in Arabidopsis. J. Exp. Bot. 2013, 64, 4183–4192.
- Li, N.; Sun, L.; Zhang, L.; Song, Y.; Hu, P.; Li, C.; Hao, F.S. Atrbohd and Atrbohf Negatively Regulate Lateral Root Development by Changing the Localized Accumulation of Superoxide in Primary Roots of Arabidopsis. Planta 2015, 241, 591–602.
- Montiel, J.; Arthikala, M.K.; Quinto, C. Phaseolus Vulgaris Rbohb Functions in Lateral Root Development. Plant Signal. Behav. 2013, 8, e22694.
- Montiel, J.; Fonseca-García, C.; Quinto, C. Phylogeny and Expression of NADPH Oxidases during Symbiotic Nodule Formation. Agriculture 2018, 8, 179.
- Tewari, R.K.; Kim, S.; Hahn, E.J.; Paek, K.Y. Involvement of Nitric Oxide-Induced NADPH Oxidase in Adventitious Root Growth and Antioxidant Defense in Panax Ginseng. Plant Biotechnol. Rep. 2008, 2, 113–122.
- Muller, K.; Carstens, A.C.; Linkies, A.; Torres, M.A.; Leubner-Metzger, G. The Nadph-Oxidase Atrbohb Plays a Role in Arabidopsis Seed after-Ripening. New Phytol. 2009, 184, 885–897.
- Li, W.Y.; Chen, B.X.; Chen, Z.J.; Gao, Y.T.; Chen, Z.; Liu, J. Reactive Oxygen Species Generated by NADPH Oxidases Promote Radicle Protrusion and Root Elongation During Rice Seed Germination. Int. J. Mol. Sci. 2017, 18, 110.
- Tanaka, A.; Christensen, M.J.; Takemoto, D.; Park, P.; Scott, B. Reactive Oxygen Species Play a Role in Regulating a Fungus-Perennial Ryegrass Mutualistic Interaction. Plant Cell 2006, 18, 1052–1066.
- Yushi, I.; Tawaratsumida, T.; Zheng, S.J.; Yuasa, T.; Iwaya-Inoue, M. NADPH Oxidases Act as Key Enzyme on Germination and Seedling Growth in Barley (Hordeum Vulgare L.). Plant Prod. Sci. 2010, 13, 45–52.
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173.
- Kai, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Watabe, G.; Yuasa, T.; Iwaya-Inoue, M.; Ishibashi, Y. Role of Reactive Oxygen Species Produced by NADPH Oxidase in Gibberellin Biosynthesis During Barley Seed Germination. Plant Signal. Behav. 2016, 11, e1180492.
- Montiel, J.; Nava, N.; Cardenas, L.; Sanchez-Lopez, R.; Arthikala, M.K.; Santana, O.; Sanchez, F.; Quinto, C. A Phaseolus Vulgaris NADPH Oxidase Gene Is Required for Root Infection by Rhizobia. Plant Cell Physiol. 2012, 53, 1751–1767.
- Arthikala, M.K.; Sanchez-Lopez, R.; Nava, N.; Santana, O.; Cardenas, L.; Quinto, C. Rbohb, a Phaseolus Vulgaris NADPH Oxidase Gene, Enhances Symbiosome Number, Bacteroid Size, and Nitrogen Fixation in Nodules and Impairs Mycorrhizal Colonization. New Phytol. 2014, 202, 886–900.
- Marino, D.; Andrio, E.; Danchin, E.G.; Oger, E.; Gucciardo, S.; Lambert, A.; Puppo, A.; Pauly, N. A Medicago Truncatula NADPH Oxidase Is Involved in Symbiotic Nodule Functioning. New Phytol. 2011, 189, 580–592.
- Kayano, Y.; Tanaka, A.; Akano, F.; Scott, B.; Takemoto, D. Differential Roles of NADPH Oxidases and Associated Regulators in Polarized Growth, Conidiation and Hyphal Fusion in the Symbiotic Fungus Epichloe Festucae. Fungal Genet. Biol. 2013, 56, 87–97.
- Nordzieke, D.E.; Fernandes, T.R.; El Ghalid, M.; Turra, D.; Di Pietro, A. NADPH Oxidase Regulates Chemotropic Growth of the Fungal Pathogen Fusarium Oxysporum Towards the Host Plant. New Phytol. 2019, 224, 1600–1612.
- Zhang, Z.B.; Zhao, Y.L.; Feng, X.B.; Luo, Z.Y.; Kong, S.W.; Zhang, C.; Gong, A.D.; Yuan, H.; Cheng, L.; Wang, X.N. Genomic, Molecular Evolution, and Expression Analysis of Nox Genes in Soybean (Glycine Max). Genomics 2019, 111, 619–628.
- Lara-Ortíz, T.; Riveros-Rosas, H.; Aguirre, J. Reactive Oxygen Species Generated by Microbial NADPH Oxidase Noxa Regulate Sexual Development in Aspergillus Nidulans. Mol. Microbiol. 2003, 50, 1241–1255.
- Dirschnabel, D.E.; Nowrousian, M.; Cano-Dominguez, N.; Aguirre, J.; Teichert, I.; Kuck, U. New Insights into the Roles of NADPH Oxidases in Sexual Development and Ascospore Germination in Sordaria Macrospora. Genetics 2014, 196, 729–744.
- Cano-Dominguez, N.; Alvarez-Delfin, L.; Hansberg, W.; Aguirre, J. NADPH Oxidases Nox-1 and Nox-2 Require the Regulatory Subunit nor-1 to Control Cell Differentiation and Growth in Neurospora Crassa. Eukaryot. Cell 2018, 7, 1352–1361.
- Roca, M.G.; Weichert, M.; Siegmund, U.; Tudzynski, P.; Fleissner, A. Germling Fusion via Conidial Anastomosis Tubes in the Grey Mould Botrytis Cinerea Requires NADPH Oxidase Activity. Fungal Biol. 2012, 116, 379–387.
- Zhang, J.; Chen, C.; Zhang, D.; Li, H.; Li, P.; Ma, F. Reactive Oxygen Species Produced via Plasma Membrane NADPH Oxidase Regulate Anthocyanin Synthesis in Apple Peel. Planta 2014, 240, 1023–1035.
- Tewari, R.K.; Watanabe, D.; Watanabe, M. Chloroplastic NADPH Oxidase-Like Activity-Mediated Perpetual Hydrogen Peroxide Generation in the Chloroplast Induces Apoptotic-Like Death of Brassica Napus Leaf Protoplasts. Planta 2012, 235, 99–110.
- Liu, B.; Zhao, S.; Tan, F.; Zhao, H.; Wang, D.; Si, H.; Chen, Q. Changes in Ros Production and Antioxidant Capacity during Tuber Sprouting in Potato. Food Chem. 2017, 237, 205–213.
This entry is offline, you can click here to edit this entry!
