Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medical Laboratory Technology
Paper-based analytical devices (PADs) have emerged as a promising approach to point-of-care (POC) detection applications in biomedical and clinical diagnosis owing to their advantages, including cost-effectiveness, ease of use, and rapid responses as well as for being equipment-free, disposable, and user-friendly. Total signal-amplification strategies in PADs involving colorimetry, luminescence, surface-enhanced Raman scattering, photoacoustic, photothermal, and photoelectrochemical methods as well as nucleic acid-mediated PAD modifications.
- paper-based analytical devices
- PADs
- signal amplification
- point-of-care diagnostics
1. Background
Point-of-care (POC) diagnostics, which play an important role in personalized healthcare, have gained considerable attention owing to operations that enable medical staff to make rapid diagnoses and tailored treatment decisions. POC diagnostics offering affordable costs, easy operations without specially trained personnel, and rapid analytical consequences that have a positive correlation with the traditional clinical laboratory, are increasingly exploited in clinical diagnostic applications, especially if medical resources are limited [1,2,3,4]. Among the types of POC testing, paper-based analytical devices (PADs) have emerged as critical POC biosensors for disease monitoring and diagnostics, particularly in resource-constrained regions, for emergencies, and for in-house healthcare owing to their advantageous features, including simplicity, easy storage, disposability, and portability without relying on external devices [5,6,7,8]. There are three main classifications for PADs—lateral flow assays (LFAs), dipstick assays, and microfluidic PADs (µPADs) [9] with diversified signal readout approaches including colorimetry [10] luminescence [11,12] surface-enhanced Raman scattering (SERS) [13,14], photothermal methods [15,16], photoacoustic methods [17], and electrochemistry [18,19]. The most common detection approach in PADs is the colorimetric method, which can easily determine the presence of a target through color variations, for recognition even with the naked eye and without complicated instruments [20]. Despite the feasibility of PADs in POC diagnostics, comprehensive applications in diagnostic biosensors have still faced several challenges, such as low sensitivity and selectivity, poor quantitative discrimination, and limited stability [9,21], which have attracted research attention aimed at addressing these issues through signal-amplification methods.
2. Colorimetric Signal Amplification
Owing to its convenience and simplicity, the colorimetric lateral flow assay (CLFA) is one of the most prevalent diagnostic technologies for point-of-care applications, particularly for the detection of biomolecules [22,23,24], metal particulates [25,26,27], or food-contaminated pesticides [28,29,30]. In principle, colorimetric detection is associated with color development caused by an enzymatic or chemical reaction that can be seen with the naked eye or a semi-quantitative device. Nevertheless, it remains a great challenge to completely eliminate the background noise generated from the sample or the paper. Furthermore, due to the heterogeneity of the color distribution, evaluation of the final color is challenging. Such a limitation in sensitivity inhibits many critical applications, such as early detection of significant cancers and severe infectious diseases. With the rapid advancements in novel materials and nanotechnology, signal-amplification strategies that hold great potential to eliminate these limitations of CLFA have been developed in recent years. Significant effort has been dedicated to increasing light output yield, including controlling the physicochemical properties of the nanoparticle markers (such as metal label size and shape [31,32,33]), functionalizing nanocomposites with polymers [34,35], or employing enzyme-mimicking noble metal NPs [36,37,38,39].
2.1. Particle Aggregation and Size Enlargement via Nucleation
One of the most common and effective ways to enhance the colorimetric signal of paper-based devices is by enlarging the particle size. The plasmonic properties of noble metal nanoparticles make them a compelling candidate for developing colorimetric devices that can significantly improve output-signal intensity, thus enhancing the sensitivity to target biological molecules. Different types of NPs have been adopted for improved signal intensity, such as gold [40,41,42], silver [43], and copper [44]. Due to their unique optical properties and ease of preparation, gold nanoparticles (AuNPs) are the most attractive material for PAD fabrication. The most widely used platform includes the deposition of metal ions (such as gold, silver, and copper) on the surface of colloidal AuNPs, in which the AuNPs catalyze the surrounding metal ions into reduced atoms. This approach was applied by Rodriguez et al. for silver and gold enhancement-based lateral flow immunoassays [43]. The enhancement of AuNPs by Au ions is based on the reduction of Au ions on the surface of existing gold nanoprobes. In the presence of hydrogen peroxide (H2O2), chloroauric acid is a reduced gold ion, enabling the formation of a new gold layer on the surface of AuNPs [45]. The silver-enhancement process is similar in principle. In particular, silver lactate or acetate is usually used as the ion source to produce the Ag+ ion. Under the catalytic activity of AuNPs at room temperature, the Ag+ ion is reduced to form large agglomerates of metallic Ag in the presence of a reducing agent (such as hydroquinone). The general principle allows the formation of silver nanoshells around colloidal gold, making its color visible [43,46]. Silver- and gold-enhancement methods allow prostate specific antigen detection at a 0.1 ng/mL limit within 20 min. However, the uncontrollable Ag shell enhancement and the lack of specificity limit its broad application. To solve this problem, several metal nanoshell preparation methods have been designed. In particular, a well-controlled nanoshell formation process was developed by adding polyethyleneimine (PEI) to the reaction to act as the capping agent to obtain a shape-controllable nanostructure. The subsequent addition of sodium ascorbate (SA) leads to reduction of the forming complex, enabling the formation of a well-defined Cu nanopolyhedron shell that is quantitatively detectable with the naked eye [47]. This approach was successfully employed by Phan et al. to prepare Cu nanoshells on AuNP surfaces in dot-blot immunoassays for sensitive detection of the Mycobacterium tuberculosis–specific antigen CFP-10 [48]. The fabrication process is illustrated in Figure 1A. First, to prepare the paper-based immunoassay, AuNPs are integrated with the CFP-10-specific antibody GBP-CFP10G2 to form GBP-CFP10G2-AuNP conjugates. Then, the conjugates are immobilized on a nitrocellulose paper strip pre-coated with the CFP-10 antigen. When a solution containing Cu2+-PEI-SA is added to the paper strip, the antibody–antigen immunoreactions cause the formation of Cu nanoshells on the surface of the GBP-CFP10G2-AuNP. This preparation procedure for Cu nanoshells on AuNP surfaces not only results in the enlarged AuNP size but also leads to a particle shape change (from spherical to polyhedral) when Cu ions are attached to its surface, thereby significantly amplifying the signal intensity. The proposed platform enables detection of the M. tuberculosis-specific antigen CFP-10 with an LOD of 7.6 pg/mL, indicating approximately 13 times more sensitivity than another AuNP-based surface plasmon resonance method. The as-prepared platform was developed by the same group using gold and copper nanoshell enhancement for highly sensitive detection of Ag85B antigen with LODs of 1.56 and 0.75 ng/mL, respectively, for gold and copper enhancement visible with the naked eye, indicating more sensitivity than the silver enhancement process [10].
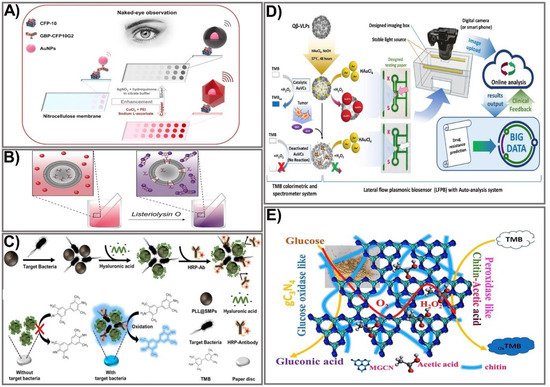
Figure 1. Strategies for signal amplification in colorimetric PADs. (A) A representation of naked-eye detection of CFP-10 using a copper/silver nanoshell enhancement-based dot-blot immunoassay platform. Adapted with permission from Ref. [48]. (B) The mechanism for detection of pore-forming toxin Listeriolysin O (LLO) using AuNP aggregation-based signal enhancement. Adapted with permission from Ref. [49]. (C) The design process and mechanism for detection of Escherichia coli O157:H7 bacteria using an enzyme functionalization-based signal enhancement strategy. Adapted with permission from Ref. [50]. (D) The principle of fabrication and detection of glutathione using peroxidase-like AuNP-based signal amplification. Adapted with permission from Ref. [51]. (E) The fabrication and detection procedure using a polymer-functionalized, metal-free dual enzyme-mimicking system to detect peroxide glucose. Adapted with permission from Ref. [37].
Owing to their ability to aggregate or disaggregate, AuNPs have also been extensively used for colorimetric immunoassays in which detection colors change from wine red to blue, an effect attributed to the agglomeration of AuNPs [52,53]. The assembly of aggregates allows an increase in the number of markers in the test zone, thereby improving the intensity of the assay. A simple and rapid sensing platform based on AuNP aggregation was reported by Mazur et al. for detection of Listeriolysin O (LLO) [49]. The design of the device was based on the formation of an LLO pore complex on cysteine-preloaded liposomes, causing the liposomes to release cysteine (Figure 1B). The freed cysteine then interacts with the AuNPs, leading to AuNP aggregation. Based on that principle, in the absence of LLO, intact liposomes and AuNPs do not interact with each other, causing the mixture to remain red. When LLO is present, AuNP aggregates formed by the interaction between released cysteine and AuNPs cause a colorimetric change from red-purple to blue, allowing for quantitative and qualitative detection of LLO. A cysteine-loaded liposome was deposited on the surface of Whatman filter paper. The moist paper was then immersed in a solution containing AuNPs. The colorimetric change elicited by AuNP aggregation in the presence of LLO enables LLO detection from as little as 12.9 µg mL−1 in PBS and 19.5 µg mL−1 in spiked human serum within 5 min, for an 18-fold enhancement in sensitivity compared to other liposome-based LLO detection assays [54]. An alternative way to prepare AuNP aggregates is based on a high concentration of salt in the reaction solution. Such a preparation method was employed by Diaz-Amaya’s group [55]. Taking advantage of salt concentration-induced AuNP aggregation, a highly stable multilayered label particle (ssDNA-PEI-Au-PS) was designed for multiple detection of mercury and arsenic. By employing PEI as an enveloping agent and using DNA aptamers as capture biomolecules, a sensitive colorimetric response was achieved in a controllable fashion. The proposed paper-based platform allows detection of mercury and arsenic at levels as low as 1 ppm in DI water and 2 ppm in river water. Other nanocomposites fabricated by conjugating different materials have also been exploited to take full advantage of the aggregation/anti-aggregation mechanism. Basiri et al. proposed an innovative and facile paper-based colorimetric assay based on AuNP-immobilized reduced graphene oxide (rGO@AuNP) nanocomposites for sensitive detection of dopamine and Cu2+ ions in human urine and tomato samples, with the detection limit reduced to 16 nM and 9.8 nM for dopamine and Cu2+, respectively [56]. Another nanocomposite, hemin-graphene nanomaterial (H-GN), which possesses tailored dispersibility in a high salt concentration and a highly active biomimetic oxidation catalyst property, was used for fabrication of a label-free colorimetric sensor for telomerase activity. The major challenges with metal NP aggregation-based signal amplification are false positive signals or a high background resulting from NP auto-aggregation, which is caused by uncontrollable external factors (for example, salt concentration, pH level, or temperature). However, with the recent development of nanomaterials and nanotechnologies, the abovementioned issues are expected to be tackled in order to improve the use of colorimetric assays in paper-based devices.
2.2. Enzyme Functionalization
With the recent achievements in nanotechnology, the employment of enzymes is a promising approach to improving signal intensity due to their remarkable catalytic properties, easy miniaturization, and substrate specificity. The utilization of enzymes for signal amplification is based on the catalytic activity of a natural enzyme that causes oxidation or reduction of a substrate, which in turn leads to sequential activation of the downstream enzymes that result in synergistic signal amplification within seconds. Currently, many multi-enzyme cascade systems have been developed, allowing rapid and sensitive detection of targets by paper-based sensing devices [57,58,59,60,61]. Another way to intensify the enzyme-labeled signal is to functionalize enzymes by linking them with nanoparticles [62]. The most commonly available enzymes used for signal amplification include horseradish peroxidase (HRP), alkaline phosphatase (ALP), and β-galactosidase. Enzyme-based signal amplification comprises the following: (1) employment of multiple enzyme complexes to accelerate the sequential enzyme reaction [63,64], or (2) tailoring of enzymes on the paper surface by nano-bioconjugates or polymers, allowing stable and function-modifiable attachment of enzymes that can significantly amplify a signal intensity that is linear to the analyte concentration, allowing for a naked-eye readout or semi-quantification with a spectrometer. Among the most common HRP substrates used in commercial immunoassays is 3,3′,5,5′-tetramethylbenzidine (TMB). An HRP-conjugated antibody and a TMB system were employed for specific signal amplification in the presence of the highly virulent bacteria Escherichia coli O157:H7 in sausages [50]. The detection platform was fabricated using magnetic separation combined with HRP-mediated signal amplification on a paper disc. The fabrication process includes the synthesis of the magnetic particle by integrating a short-chain glucan (SCG) with iron oxide NPs pre-coated with dextran (Dex@IONPs) to form a starch magnetic particle (SMP). The SMP surface was then functionalized with poly-L-lysine into the PLL@SMP composite, which was then added to the sample solution containing bacteria at different concentrations, followed by incubation with HA. For specific capture of bacteria, an HRP-conjugated bacterial specific antibody was used. The detection areas were prepared by soaking the paper discs in the TMB solution and allowing them to dry. Finally, the TMB-coated paper discs were immersed into the antibody-captured bacterial solution, and color change upon the reaction of HRP and TMB detected the bacterial concentration. Hyaluronic acid was used as a blocking agent to increase sensitivity and minimize the background signal (Figure 1C). The capture efficiency of the synthesized PLL@SMP-based platform for pathogenic bacteria was calculated at higher than 90%. The proposed sensing system was successfully used to detect the target pathogenic bacteria E. coli O157:H7 in sausage samples with an exceptionally low LOD of 30.8 CFU/mL, an approximate 300-fold enhancement in comparison to the conventional paper-based detection platform [65].
Besides HRP, alkaline phosphatase, glucose oxidase (GOx), and β-galactosidase also show significantly enhanced colorimetric signals on sensing platforms. Notably, ALP has been employed as a signal-amplification enzyme in many colorimetric immunoassays, owing to enzymatic activity that catalyzes the dephosphorylation or transphosphorylation of phosphate molecules [66]. Another commonly employed enzyme is GOx, which is used for glucose-level assessment. GOx can catalyze the oxidation of glucose to produce H2O2, which in turn is the substrate of the next enzyme-catalyzed reaction. Recently, GOx has been coupled with other enzymes, such as HRP, to design colorimetric immunoassays with significantly improved color development [60,67]. The current progress in enzyme functionalization strategies has seen significant advances in developing new and sensitive enzyme-aided paper-based sensing devices, providing promising sensing devices for sensitive detection of various molecules.
2.3. Metal Nanozyme Modification
Due to their high catalytic activity and stability, nanostructured artificial enzymes known as nanozymes have been considered effective alternatives to natural enzymes, offering attractive advantages. Compared to natural enzymes, nanozymes possess distinct advantages, including robustness, high catalytic activity, low cost, good stability, and easy mass production [68,69]. A number of nanomaterials with various compositions and nanostructures have been successfully synthesized, and they possess highly catalytically enzyme-like activities, including oxidase-, peroxidase-, and catalase-like nanozymes. Various well-controlled bimetallic NP-based nanozyme composites have been successfully used to enhance signals by mimicking natural peroxidases when integrated into colorimetric PADs. In principle, nanozyme-based colorimetric assays are based on the catalytic activity of the nanozyme toward chromogenic substrates, giving rise to color change that is correlated with the target concentration. Among the various NPs, AuNPs have exhibited excellent enzyme-mimicking activity, including peroxidase- and GOx-like activity. The peroxidase-like activity of gold-viral biomineralized nanoclusters (AuVCs) has also been successfully exploited as a nanozyme for the design of a lateral flow plasmonic biosensor (LFPB) for on-site glutathione (GSH) determination visible to the naked eye that has been quantified by auto-analysis software (Figure 1D) [51]. Initially, bioproduction of Qβ virus-like particles (VLPs) was prepared via transformation of the Qβ coat protein genome (pCDF–QβCP) into E. coli, enabling self-assembly of the QβCP into VLPz, which was then incubated with AuNPs in the presence of HAuCl4 and NaOH to generate AuNCs. The as-prepared AuVCs exhibited a high capacity when used to catalyze the formation of AuNPs in the presence of HAuCl4 and H2O2. In the presence of increased target analyte GSH, the AuVCs were deactivated, causing decreased catalytic activity, and a resultant reduction of AuNP formation was achieved, allowing for determination of the GSH concentration. Testing paper that consisted of recognition lines and flow-detection zones was printed from a wax printer. The sample was captured by an image box with a smartphone or digital camera and sent to online auto-analyzing software. Under optimized conditions, grayscale values plotted against GSH concentrations exhibited a linear relationship within the range 25–500 µM (LOD 9.80 µM), which is about a six-fold improvement compared with another paper-based electroanalytical test strip [70], with a highly positive correlation between the detected GSH level and the temozolomide (TMZ) drug-resistance level in glioblastoma multiforme (GBM) cells. Being oxidase-like is another good activity exerted by metal oxides that have been employed for signal improvement in several paper-based devices. Taking advantage of oxidase-like degradable manganite nanowires (γ-MnOOH NWs), a facile colorimetric paper sensor using γ-MnOOH NWs as a degradable nanozyme and TMB as a chromogenic indicator was developed for rapid and sensitive screening of organophosphorus pesticides (OPs) and acetylcholinesterase (AChE) [71]. The sensing mechanism was based on the oxidase-like activity of γ-MnOOH NWs in the presence of TMB as a chromogenic substrate. The reaction of acetylcholinesterase–acetylthiocholine (AChE-ATCh) releases thiocholine (TCh) that subsequently causes the degradation of γ-MnOOH NWs into an invalid Mn(II) ion, which could be used as a recognition element for AChE inhibitors (OPs). Degradation of the γ-MnOOH nanozyme leads to a significant loss of enzymatic activity toward TMB oxidation, thereby reducing color development. Based on that mechanism, the concentration of OPs could be measured by the color change of the TMB product at absorbance wavelength 652. The as-prepared detection platform was integrated on test paper, achieving higher sensitivity compared to an acetylcholine-conjugated paper test strip [72]—LOD 0.1 mU mL−1 for AChE activity, 10 ng mL−1 for omethoate, and 3 ng mL−1 for dichlorvos in real serum and vegetable samples.
2.4. Conjugated Polymer Functionalization
Owing to their high biocompatibility, biodegradability, and chemical and environmental stability, polymers have been used for NP modification to improve mechanical, thermal, electronic, or optical properties. Recent advances have focused on the fabrication of flexible polymer nanocomposite-based sensing devices that have shown wide applicability with high sensitivity. Nanocomposites functionalized with polymers could greatly improve mechanical, physical, and optical properties as well as multi-functionality. In paper-based colorimetric detection platforms, the integration of a polymer with nanomaterials has been successfully used for significant signal improvement. Different types of polymers have been employed for paper-based sensor fabrication. Chitin, a natural polysaccharide, was recently used as a bridge between graphitic carbon nitride and acetic acid to obtain glucose oxidase and peroxidase mimics [37]. Taking advantage of glucose oxidase-like modified graphitic carbon nitride (MGCN) and peroxidase-like chitin–acetic acid (chitin-AcOH), Sengupta and co-workers developed an MGCN-chitin-AcOH nanocomposite in a paper test strip through immobilization of MGCN-chitin-AcOH and a TMB substrate using a polyvinyl alcohol hydrogel composite (Figure 1E). To achieve glucose oxidase-like activity, GCN was chemically modified by a calcination procedure. Subsequently, the hybrid MGCn-chitin-AcOH was prepared by a control fusion technique, allowing the formation of a biofunctional nanozyme. MGCN-chitin-AcOH, when in contact with glucose, oxidized glucose to gluconic acid and hydrogen peroxide, while chitin-AcOH decomposed the generated H2O2, as proved separately by concurrent oxidation of TMB. The developed method was successfully applied to detect H2O2 and glucose in human serum and urine with a low detection limit of 0.052 μM for H2O2 and 0.055 μM for glucose.
3. Luminescent Signal Amplification
Among the optical biosensors, luminescence-aided sensing, including chemiluminescence and bioluminescence, is a particularly compelling approach to signal transduction in many chemical sensing schemes due to the higher signal-to-noise ratio and the simplicity of the required measurement equipment. In general, detection with a luminescence-based device can be achieved by imaging or measuring the light emitted by bio-chemiluminescence or electro-generated chemiluminescence. Much effort has been dedicated to achieving an enhanced luminescence signal in PADs, mainly focusing on the choice of appropriate labeling agents, including the use of enzymes as labels in conjugation with enhancers to obtain improved signal intensity [73], metal-enhanced chemiluminescence [74], artificial pseudo-enzyme labels [75], and modified nanomaterial-based signal on-off mechanisms [76]. The use of nanomaterials has shown great promise in producing high-performance sensing devices due to their ability to act as the novel label of luminescent detection as well as being a platform to enhance the loading capacity of the luminescent labels. Liu’s group developed a novel dual-key-and-lock strategy-based ruthenium (II), or Ru(II), complex probe (Ru-FA) as an effective tool for formaldehyde detection in vitro and in vivo [77]. Ru-FA showed weak luminescence due to the photon-induced electron transfer (PET) process from the Ru(II) center to electron-withdrawing group 2,4-dinitrobenzene (DNB). Triggered by a specific reaction with formaldehyde (the first key) in an acidic environment (the second key), DNB is cleaved from Ru-FA, affording an emissive Ru(II) complex derivative, Ru-NR (Figure 2A). As a result of the PET process from the Ru(II) center to electron-withdrawing moiety DNB, Ru-FA itself displays weak luminescence, but its emission can be significantly increased after reacting with formaldehyde (key 1) in an acidic environment (key 2), accompanied by the production of emissive Ru-NR. This proposed system allows detection of formaldehyde at a 19.8 nM LOD, which is approximately 15 times higher than other paper-based analytical devices [78]. The on-off state of paper-based valves controlled by the rotation of paper discs is another strategy that has been used recently for improving a luminescence signal [79]. The rotational paper-based device was fabricated by assembling three designed paper discs using a hollow rivet. The on-off state of paper-based valves was easily controlled by rotation of the paper discs (Figure 2B). The integrated paper-based rotation valves can easily be controlled by rotating the paper discs manually, making it user-friendly for the untrained. In addition, the rotational valves are reusable, and the response time can be shortened to several seconds, which promotes the rotational paper-based device as offering great advantages in multi-step operations. Under the control of rotational valves, multi-step ECL immunoassays were conducted on a rotational device for multiplexed detection of carcinoembryonic antigen (CEA) and prostate specific antigen (PSA). The rotational device exhibited excellent analytical performance for CEA and PSA, which could be detected in linear ranges of 0.1–100 ng mL−1 and 0.1–50 ng mL−1 with limits as low as 0.07 ng mL−1 and 0.03 ng mL−1, respectively, which is approximately 10 times more sensitive than a paper-based fluorometric device [80]. Compared to other, conventional paper-based valves, the as-prepared platform could be dried for reuse, revealing its simplicity, rapidity, low cost, and excellent analytical performance. Due to its diverse structural polymorphism and an ability to switch on the signal upon binding to luminescence molecules, the non-canonical DNA secondary structure is another effective way to improve a luminescence signal. Sun et al. developed a paper-based µPAD based on a G-quadruplex-based luminescence switch-on assay for detection of the lead(II) ion (Pt2+) [81]. This type of suspended-droplet mode, paper-based µPAD uses wetting and gravity as a driving force. To fabricate the super-hydrophobic pattern on a paper device, a new microcontact printing-based method was applied by coating hydrophobic and transparent silicone polydimethylsiloxane (PDMS) on a glass slide attached with Teflon (a non-stick polymer allowing easy peel-off of the PDMS). For Pt2+ detection, G-quadruplex oligonucleotide and the iridium (III) probe, respectively, were added to the reaction zone and the detection zone of the test strip. The presence of Pt2+ allowed conformational change in the G-quadruplex, which can greatly enhance the luminescence emission of the iridium (III) probe (Figure 2C). The proposed platform was integrated into an inexpensive, battery-powered compact device for routine portable detection using a smartphone. Pt2+ was detected at low concentrations within the linear range from 10 nM to 100 nM. Excited-state proton transfer (ESPT) with huge luminescence Stoke shifts and an ultrafast response is another way to enhance the luminescence signal intensity and has generated great interest. Recently, an ESPT concept-based luminescence sensor was designed for discriminative detection via enol-keto tautomerism [82]. To improve the sensitivity, two-dimensional (2D) nanosheets of a metal-organic framework (MOF), Cd2(2,5-tpt)(4,5-idc)(H2O)4, were synthesized via top-down liquid ultrasonic exfoliation technology for sensing water in dimethylformamide (Figure 2D). This sensor can serve as a dual-sensing mechanism along with luminescence color change via shifted emission (green to yellow) in low water content and can be a turn-off method in high water content. Such a 2D nanosheet-sensing platform was applied to a paper test strip for ease of water detection with a rapid response (<30 s), long-term stability, pH stability, good reusability, high selectivity, broad-range detection (0–50% v/v), and a low LOD value (0.25% v/v) that is considerably lower than the conventional MOF-based sensing platform [83]. The recent improvements in materials and nanotechnologies have created new opportunities to further enhance luminescence signals through numerous strategies, including the use of proper labeling agents, a choice of fabrication process, or the use of an electrical energy supply. These approaches have shown great promise in producing handheld, paper-based devices with more sensitive detection of analytes using miniaturized devices. With a comprehensive understanding of the luminescent PAD, new generations of these sensing systems promise to solve the limitations of previously established devices, and could be employed in commercial applications.
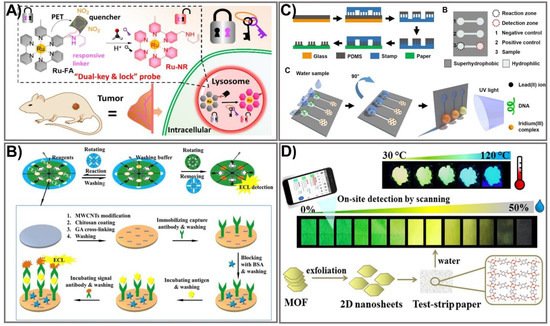
Figure 2. Luminescence-based PADs with enhanced detection sensitivity. (A) The schematic of the PAD assay for lysosomal formaldehyde detection using dual-key-and-lock ruthenium (II) complex probe as a signal amplification strategy. Adapted with permission from Ref. [77]. (B) Schematic illustration for the implementation of 3 paper discs in a rotational paper-based valve for a multi-step electrochemiluminescence immunoassay for the detection of carcinoembryonic antigen and prostate-specific antigen cancer markers. Adapted with permission from Ref. [79]. (C) Schematic representation of the suspended-droplet mode-based luminescence PAD design process for the detection of lead (II) ions in water samples. Adapted with permission from Ref. [81]. (D) The schematic principle of the excited-state proton transfer concept-based luminescence PAD for water and temperature sensing. Adapted with permission from Ref. [82].
4. Surface-Enhanced Raman Scattering Signal Amplification
SERS is a sensing technique that generates and amplifies inelastic light scattering of molecules when they are adsorbed on metals like gold, silver, and copper. This scattering signal could be enhanced (up to 10 orders of magnitude) by modulating the frequency of excitation light and the localized surface plasmon resonance (LSPR) of the metallic nanomaterials, relevant to the Stoke and/or anti-Stoke lines of molecules. In a SERS-based assay, specific tags made of nanostructures, and molecules with known Raman fingerprints, are the detection agents. Measuring the peak intensity of Raman molecules allows for quantification of particle and analyte concentrations. Consequently, SERS has become a powerful tool in diagnostics due to its rapid, sensitive, and multiplexed outcomes.
Manipulating the shape, size, and composition of the nanostructure has shown great promise in improving the SERS signal. In particular, the gold nanostar (GNS) providing high SERS performance was used as a Raman reporter for PAD fabrication that showed significant signal intensity in the detection of bisphenol A (BPA) [84]. Gold nanostars with a sharp branched shape are great material for tuning plasmonic properties, providing the strongest SERS activities. GNSs were used as a SERS nanotag in comparison with spherical AuNPs. Both GNSs and AuNPs were tagged with the Raman reporter molecule ATP, followed by incubation with an anti-BPA antibody to form GNS/AuNP-antibody-ATP conjugates. BSA was subsequently used as a blocking agent, and 4,4-bis(4-hydroxyphenyl) valeric acid (BHPVA) was allowed to be bound by BSA to prepare the BHPVA-BSA conjugate. An LFA strip was used, consisting of a nitrocellulose membrane, a conjugate pad, an absorbent pad, a sample pad, and a support sheet. On the conjugated pad, the GNS/AuNP-ATP-antibody conjugate was dispersed, and the nitrocellulose membrane was coated with BHPVA-BSA and a goat anti-rabbit antibody as a control. For BPA detection, the BPA solution was dropped on the sample pad, where the analyte migrates to the membrane and conjugate pad, and finally moves to the absorbent pad. The color change in correlation with BPA concentration caused by the presence of GNS allowed quantitative detection of BPA (Figure 3A). The as-prepared SERS-LFA allows BPA detection at concentrations ranging from 0.05–60 ppt with a detection limit down to 0.073 ppt, indicating it is about 136 times more sensitive than the PEGylated AuNP-based LFA [85]. An alternative strategy for SERS enhancement is multilayer nanostructure fabrication that takes advantage of all employed compositions. As such, a SERS paper-based sensor was constructed by decorating paper chips with a sandwich structure of 3D silver dendrite (SD)/electropolymerization of molecular identifier (EMI)/silver nanoparticle (AgNP) (Figure 3B) [86]. The SD was synthesized on the surface of paper chips using a chemical reduction growth technique followed by immobilization of the EMI layer that serves as a specific recognizer. Subsequently, the core enhancement AgNP layer was integrated on top of the sensing chip. The as-prepared SERS paper chip was successfully applied for in situ detection of imidacloprid (IMI), showing ultra-high sensitivity with a detection limit of 0.02811 ng mL−1, 2000 times more sensitive than other paper-based organic–inorganic manganese (II) halide hybrid sensors [87]. This multiple SERS enhancement paper chip holds great potential for screening a variety of contaminants. The plasmonic alloy of different noble metals is another effective way of providing high-quality opportunities for tuning plasmon resonance. Taking advantage of the hotspot effect that creates an intense electromagnetic field near the plasmonic structure, the plasmonic alloy has been used for sensitive biosensor techniques such as SERS. Recently, a paper-based plasmonic substrate with a plasmonic alloy of Au/Ag nanocomposites on hierarchical cellulose micro-/nanofiber matrices for highly sensitive metal-enhanced fluorescence (MEF) and SERS biosensor applications was developed [88]. In the fabrication process, Au and Ag were deposited on the cellulose fibers at a low-temperature wafer level below 100 °C to avoid damage to the paper substrate and cellulose fibers (Figure 3C). The Au/Ag nanocomposite was thermally evaporated from the surface of the cellulose fiber. The integration of Au/Ag nanocomposites that act as a plasmonic alloy allows two distinct extinction peaks from Au and Ag to combine into a single peak. This paper-based plasmonic alloy substrate enables a two-fold increase in fluorescence signals and selective MEF signals, compared to Whatman chromatography paper. The proposed sensing device allows detection of folic acid at a picomolar level (LOD 1 pM), which is 1000 times more sensitive than other carbon dot-based paper devices (0.28 µmol/L) [89]. Paper-based devices have also been fabricated for the detection of foodborne bacteria. However, the low intensity of conventional Raman spectroscopy and fluorescence interference makes it difficult to detect biological samples in the complex. One strategy to deal with this challenge is to develop three-dimensional (3D) SERS substrates that offer a larger surface area for absorbing more probe molecules, and that generate more hotspots for analyte binding. Such an idea was applied to developing a novel, label-free, filter paper-based 3D-SERS detection platform to detect and classify common foodborne bacteria, including E. coli, Listeria monocytogenes, and Staphylococcus aureus [90]. Black phosphorus-Au (BP-Au) nanosheets were used as a SERS substrate that could generate abundant hot spots, showing great potential to serve as excellent SERS substrates (Figure 3D). The BP-Au sheets were prepared by conjugating AuNPs on prepared BP sheets to form BP-Au sheets. Subsequently, the BP-Au sheets were applied to the filter paper surface. This BP-Au filter paper-based 3D-SERS substrate exhibited remarkably improved Raman signals, allowing for specific recognition and classification of three types of target bacteria at concentrations as low as 10−7 CFU/mL, with an enhancement factor of 2.4 × 104 (17 times higher than the Au/Ag paper substrate) [91]. This paper-based sensing device would be beneficial in practical applications for food safety. The employment of enzyme-like noble metal NPs as substrates for SERS is another approach that significantly improves SERS signals. Taking advantage of nanozymes for the catalytic degradation of methyl mercury, several paper-based SERS sensing devices using nanozymes have been developed. In particular, Liu et al. reported fabrication of Au-NiFe-layered double hydroxide (LDH)/rGO nanocomposites, which are not only efficient nanozymes with oxidase-like activity but also efficient SERS substrates to determine organic mercury [92]. Under the free radicals of electron paramagnetic resonance (EPR) spectra and the binding energy of an X-ray photoelectron spectrometer (XPS), upon the oxidase-like catalytic reaction, the oxygen molecules are captured, leading to increased O2 radicals, while MeHg is degraded, resulting in the release of CH3 radicals. The as-prepared Au-NiFe LDH/rGO nanocomposite could be used to effectively degrade and remove 99.9% of organic mercury in two h without secondary pollution of Hg2+, and a low concentration of MeHg was detected (as low as LOD 1 × 10−8 M), which is 10 times more sensitive than the conventional paper-based device [93]. To date, much effort has been devoted to enhancing the signal output for SERS-based PADs. The innovative achievements in the bioscience and nanomaterial science fields hold great promise for boosting fabrication of highly sensitive, low-cost, and miniaturized devices for point-of-care applications.
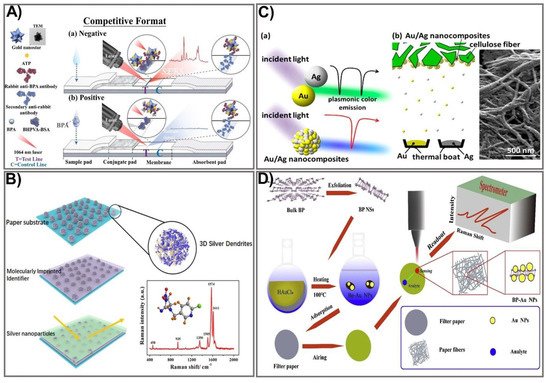
Figure 3. SERS-based signal amplification strategies for PADs. (A) The schematic principle of a gold nanostar-based SERS substrate for the sensing of biphenol A. Adapted with permission from Ref. [84]. (B) The design process for a multilayer SERS paper substrate for neonicotinoid pesticide sensing. Adapted with permission from Ref. [86]. (C) Schematic showing the design of a plasmonic alloy Au/Ag nanocomposite-based SERS substrate for signal enhancement in PAD-based biomarker sensing. Adapted with permission from Ref. [88]. (D) Representation of the design for a BP-Au filter paper-based SERS substrate for the detection of foodborne bacteria. Adapted with permission from Ref. [90].
5. Photothermal Signal Amplification
The photothermal activity of nanomaterials is another exciting property that has been exploited in recent years for the enhancement of paper-based sensing assays. Generally, the application of photothermal agents in the field of bioanalysis is based on light-to-heat conversion, which can be significantly intensified by using appropriate photothermal agents and a proper recognition system. Consequently, photothermal agent-mediated detection of a target can be carried out using a thermometer as a readout where the temperature signal is linear in relation to the target concentration. A wide variety of materials, including AuNPs [94], carbon NPs [95], quantum dots [96], and plasmonic NPs [97], have been exploited as photothermal agents for broad application in various fields. AuNPs that exert excellent photothermal signals have been extensively investigated to improve sensitivity [98,99]. Recently, a dual-signal biosensor based on multifunctional MnO2-Au was developed, enabling rapid achievement of qualitative information (visible to the naked eye) and quantitative photothermal data when detecting furazolidone antibiotic residues [100]. The fabrication process includes functionalization of AuNPs with MnO2 nanoflowers to form MnO2-Au signal probes that exhibit high colorimetric/photothermal properties. The MnO2-Au probe is then conjugated with a specific antibody (anti-CPAOZ mAb), followed by incubation with BSA to block non-specific binding. MnO2-Au exhibits a remarkably enhanced light-to-heat conversion effect attributed to the MnO2 nanoflowers’ high capacity to carry AuNPs. The flow immunoassay strip was composited from a nitrocellulose membrane, an absorbent pad, a sample pad, and a conjugate pad. Consequently, AOZ (the metabolite of FZD) can be quantitatively measured on the basis of both color change and heat signal (Figure 4A). The as-prepared immunoassay allows sensitive and comprehensive detection of FZD in food at low levels, attributed to the excellent light-to-heat conversion capacity of the MnO2-Au signal probe. FZD was detected at LOD 1 ng mL−1 and 0.43 ng mL−1 by colorimetric signal measurement and photothermal signal, respectively, indicating eight-fold improved sensitivity compared to the conventional colorimetric, gold-based lateral flow immunoassay [101]. The excellent performance exerted by this sensing device would be a promising sensing platform for broader application in point-of-care settings.
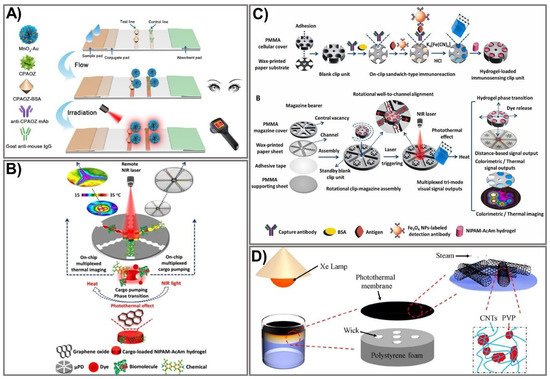
Figure 4. Schematic design of an amplified PAD signal using photothermal-based techniques. (A) Schematic representation of the MnO2-Au-based dual-signal immunoassay for AOZ detection. Adapted with permission from Ref. [100]. (B) Principle of photothermal microfluidic pumping-based PAD fabrication. Adapted with permission from Ref. [102]. (C) The design of the clip-magazine-assembled photothermal biosensing disk for tri-mode visual quantification of prostate-specific antigen. Adapted with permission from Ref. [103]. (D) Schematic demonstration of CNT@PVP photothermal membrane-based solar steam-generation device fabrication for water desalination. Adapted with permission from Ref. [104].
Recently, numerous advances in microfabrication techniques have been made, offering more facile and sensitive devices that possess many advantages over the conventional approaches. The microfluidic platform-based photothermal sensing assay is one of the currently emerging systems that hold great potential for paper-based point-of-care testing [102,105,106]. Microfluidic technologies have become increasingly powerful tools for developing point-of-care diagnostics because of their distinct advantages. However, the conventional microfluidic devices fabricated on silicon or glass surfaces require intricate design processes with complex instruments, limiting their practical application [107]. On the other hand, paper-based microfluidic devices exhibit great properties, including low cost, simplicity, sensitivity, and portability, making them a promising tool for point-of-care testing. The integration of the microfluidic platform with an intelligent sensing element is a novel strategy to compensate for the limitations of conventional microfluidic devices through synergistic performance in a single pattern. A photothermal microfluidic pumping approach was developed for an on-chip µPAD in a spatiotemporally controllable and contactless manner [102]. A multiplexed cargo reservoir fabricated from thermo-responsive poly(N-isopropylacrylamide)-acrylamide hydrogel-doped graphene oxide (GO) was immobilized on a paper surface (Figure 4B). The integration of composite hydrogels on highly photothermal-capable GO resulted in an on-chip phase transition of the composite hydrogels in a switch-like fashion. As a consequence, a strong pumping dynamic of the cargos from hydrogel to the reaction zones was achieved. By remotely controlling the laser power, GO density, and irritation time, the microfluidic pumping performance could be spatiotemporally manipulated. To demonstrate the efficiency of this pumping strategy, horseradish peroxidase and FeCl3 were used as the model cargoes to immobilize 3,3′,5,5′-tetramethylbenzidine and Prussian blue, respectively, as photothermal probes for µPAD-based colorimetric reactions. Owing to its high integrability in lab-on-chip paper-based devices, its controllable spatiotemporal capacity, and its features of being contactless and highly flexible, this novel microfluidic pumping platform shows great potential for numerous microfluidic applications.
Nevertheless, without the assistance of biological materials, photothermal sensing devices show remarkable limitations when applied to bioanalysis, including immunoassays. To overcome this challenge, Fu et al. developed a photothermal biosensing approach by integrating a photothermally responsive poly methyl methacrylate (PMMA)/paper hybrid disk (PT-Disk) in a clip magazine-assembled fashion [103]. The clip units consist of a honeycomb-patterned paper substrate and a hydrogel carrier fabricated from a PMMA cellulose cover conjugated with a specific antibody. These clip units were rotationally assembled on a magazine bearer composed of a strip-formed, channeled-paper sheet and an upper PMMA cover. To accelerate the photothermal conversion efficiency, Fe3O4 was captured on the paper substrate with an antibody and transported to a dual-functional probe—Prussian blue (PB) NPs that exert both colorimetric and photothermal activities. Subsequently, the dye-enveloped thermo-responsive hydrogels were loaded in clip wells. Upon laser irradiation, the photothermal effect of the PB NPs stimulated a rise in the dye solution’s temperature in a dose-dependent fashion, leading to the release of dye solution from the clip units to the surrounding magazine-bearing channels, which could be quantified by on-chip rulers and a portable camera (Figure 4C). The as-proposed sensing device allows analyte detection in a tri-mode signal output approach, including colorimetric readout on the paper disk for thermal-image and distance-reliable visual quantification. Using this novel sensing platform, the cancer biomarker (a prostate-specific antigen) was detected at LOD 1.4–2.8 ng mL−1, which was lower than the previously reported practical diagnostic threshold [108,109].
Due to their unique properties, photothermal-based devices have been employed for broad application in various fields, including controlling evaporation rates under solar irradiation. The hydrophobic surface is well-known for the design of water treatment systems. However, interfacial thermal resistance events occurring after hydrophobic treatment usually lead to remarkably low energy efficiency, limiting the water treatment process. To address this limitation, a carbon nanotube (CNT)-modified superhydrophobic photothermal membrane was recently fabricated on a filter paper substrate for effective water desalination upon solar irradiation [104]. In particular, the superhydrophobic photothermal membrane was composited from the CNT and polyvinylpyrrolidone (PVP) modified with 1H,1H,2H,2H-perfluorodecyl (CNT@PVP). The CNT@PVP composite was sprayed on a filter paper surface to produce a photothermal membrane that is impermeable to water. The membrane was placed onto an expanded polystyrene-prepared bottom stand for flexible floating and heat localization (Figure 4D). The as-prepared device exhibited high energy efficiency (91%) with an increased evaporation rate of 1.41 kg m−2 h−1, indicating higher desalination efficiency than previously established devices [110,111]. The key advantages of the photothermal conversion system include a high light-to-heat capability, reusability, stability, low cost, and portability, making it a promising tool for signal amplification and quantification of PADs.
6. Photoacoustic Signal Amplification
Photoacoustic (PA)-enhanced signals have been developed in a few PAD-based biosensing platforms for cryptococcal antigen [112], heparin [113], and ALP [114]. Photon energy, strongly absorbed by chromophores, is converted into mechanical energy via thermal expansion, resulting in the production of acoustic waves as PA signals [115,116]. Zhao et al. developed photoacoustic-based LFA using AuNPs to quantitatively measure cryptococcal antigen (Figure 5A). Compared to semi-quantitative colorimetric analysis, the PA detection method improves the sensitivity of LFAs owing to the strong LSPR of AuNPs and the effective elimination of a PA background signal associated with ambient light noise. Light absorption was specifically designed to enhance the PA signal with effective minimization of the background signal from paper substrate. To confirm the enhanced effect of the PA method on LFA, the limit on detections measuring cryptococcal antigen was calculated for three different measurement methods, including colorimetric measurements (1.1 ng/mL), chop mode PA detection (0.57 ng/mL), and scan mode PA detection (0.010 ng/mL), indicating the capability of the PA method to decrease LOD by more than 100 times. This PA-based LFA offers advantages in terms of high sensitivity, reduced system noise, strong and reliable PA signal generation from AuNPs, and minimization of expensive photodetectors and optical filters [112]. Another paper-based photoacoustic sensor for direct detection of heparin was fabricated for point-of-care measurement of heparin activity in human blood samples via fingerprick-sized blood samples (Figure 5B). A cellulose-based photoacoustic heparin sensor was fabricated by loading cationic dye (Nile blue A) onto polyethylene glycol (PEG)-modified Whatman filter paper. Heparin, a negative-charged polysulfated glycosaminoglycan, can interact with Nile blue A to exhibit PA spectral alternation. This PA-based sensor showed LOD at 0.28 U/mL of heparin in human plasma with a turnaround time of 3 min and at 0.29 U/mL in whole blood within 6 min. The correlation of heparin, turnaround time, and sample requirement is comparable to an activated clotting time test. This PA sensor for heparin can monitor not only heparin concentration but also heparin activity in human serum samples, which is exploited as an affordable and disposable heparin sensor [113]. Instead of AuNPs, Zhang et al. developed a portable photoacoustic device for determination of ALP in serum using silver nanoparticles with excellent photo-stability and reproducibility (Figure 5C). The breakdown of sodium L-ascorbyl-2-phosphate into ascorbic acid was catalyzed by the ALP enzyme, thereby converting AgNO3 into AgNPs. Under irradiation from a modulated 638 nm laser, a strong PA signal generated by AgNPs due to their localized plasmon resonance was detected by the portable PA device. After optimizing the reaction condition, this PA-based sensor could detect ALP in a concentration range of 5–70 U/L with LOD of 1.1 U/L. This rapid portable PA device exhibits favorable reliability and stability for determination of ALP in human serum with high sensitivity, providing a PA-based analytical strategy to detect disease-correlated biomarkers [114]. Although the reproducibility of PA-based PADs should be thoroughly validated due to the intrinsic point-scanning reading of the PA technique, the PA technique may exhibit promising potential to measure biomarkers in clinical realities.
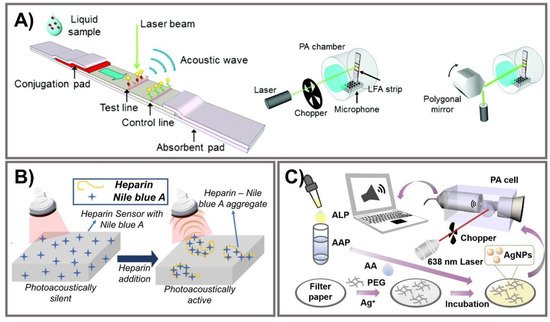
Figure 5. Photoacoustic signal amplification. (A) From left to right, PA-based LFA strip illuminated by a laser beam to generate PA signals; illustration of PA measurement systems for chop mode and scan mode. Adapted with permission from Ref. [112]. (B) Illustration of a heparin sensor via interactions with Nile blue A to activate photoacoustic signals. Adapted with permission from Ref. [113]. (C) PA device for ALP detection via PA signal generation of AgNPs from hydrolyzed AAP on filter paper. Adapted with permission from Ref. [114].
7. Photoelectrochemical Signal Amplification
Photoelectrochemical (PEC) detection has drawn increasing attention due to its promising analytical features, including low cost, simple equipment, portability, persuasive selectivity and sensitivity, and low background, facilitating high-throughput and quick assays [117,118,119,120]. Driven by these advantages, paper-based PEC systems that integrate the inherited excellence of cellulose paper and PEC bioanalysis have been fabricated [117]. The PEC platform stems from the electrochemical ideal, which can convert light energy into chemical and electrical energy, hence ameliorating sensitive detection through the modification of photocurrent responses into photoelectrochemical reactions based on analytes and specific photoactive matrix/probe interactions [117,121,122]. Photoactive electrons can be stimulated upon excitation by light, and conveyed to an electrode to generate a vigorous photocurrent signal, for which a signal-off or signal-on readout method is employed [117,122].
Despite outstanding achievements that have been obtained in lab-on-paper analytical devices, certain challenges for employed electrodes in assisting paper-based devices still exist. First, the most serious reason is associated with conductive inks that only cover the paper’s surface and are not assembled into the interior structures, leading to limited conductivity and, eventually, considerable influence on the sensitivity of the devices. The next problem is associated with practical stability under harsh conditions due to too-weak interactions between cellulose fibers and conductive materials. Finally, highly efficient lab-on-paper analytical devices urgently require remarkable capacity to generate, transmit, and measure electrical signals [123]. Therefore, PEC-based signal amplification enhancement that can address these issues is necessary in order to minimize the number of assays, but still reach high sensitivity with a very low concentration. In the last few years, diverse strategies have been proposed to amplify photoactive signals for PEC-sensing fabrication, most of which have been employed in electron acceptor/donor regulation [120,123,124,125,126], photoactive materials, nanozymes [119,120,127,128,129,130,131,132,133,134,135], or light sources [136,137,138,139,140,141,142]. Among them, functional nanomaterial integration is an emerging potent route to reach giant, efficient lab-on-paper analytical devices, and diverse photoactive nanomaterials have been immobilized on cellulose fibers. Although it is well known that an excited light source is requisite for a photoelectrochemical platform, physical light source-related instruments are also required [142]. Therefore, to achieve instrument miniaturization as well as operational simplification, luminol-based chemiluminescence emission was exploited as an interior light source, which offers multitudinous advantages, including low cost, simplicity, high sensitivity, and a rapid response [136]. Lan et al. designed and constructed a novel 3D-rGO/cellulose-based photoelectrical lab-on-paper analytical device to determine thrombin (TRB) in serum samples, a procedure typically utilized for Alzheimer disease and cardiovascular disease diagnosis [142]. The combination of reduced graphene oxide and Au flower in paper cellulose fiber facilitates superior conductivity as well as biocompatibility. ZnO anchored/nitrogen-doped carbon dots are immobilized in this system and stimulated by strong chemiluminescence of a luminol–H2O2 system to generate a photocurrent signal (Figure 6A). This proposed sensing performed with excellent sensitivity, as well as specificity in thrombin determination, and can detect very low concentrations of 16.7 fM.
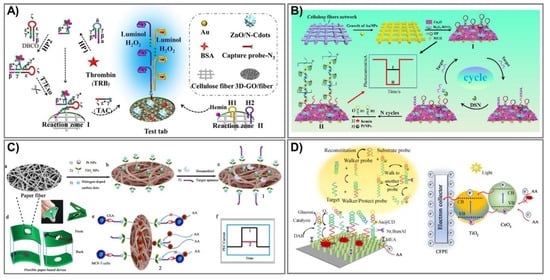
Figure 6. Photoelectrochemical paper-based analytical devices. (A) The intrinsic light Au@3D-rGO/cellulose sensor generates a photocurrent signal to detect protein in clinical biomedicine. Adapted with permission from Ref. [142]. (B) The construction process and mechanism for cathode PEC PADs shown to detect microRNA-141 by duplex-specific-nuclease (DSN) connection. Adapted with permission from Ref. [143]. (C) The PEC TiO2–Pt/PWE biosensor was fabricated by modification of paper with N-carbon dots/TiO2–Pt with a seed-mediated growth method to detect carcinoembryonic antigen (CEA). (a–c) preparation of photoelectrochemical biosensor, (d) diagram of paper-based platform, (e) detection mechanism, (f) photocurrent intensity with/without CEA. Adapted with permission from Ref. [144]. (D) An ultrasensitive PEC paper-based device to determine miRNA-141 from a two-enzyme-engineered DNA walker and a TiO2/CeO2 heterojunction. Adapted with permission from Ref. [128].
Alternatively, sensing can obtain the dual functions of target recognition and signal transduction, which are widely employed in cyto-assays due to their triggering of the signal of target recognition [123]. Recently, the electron acceptor/donor methodology has been widely investigated for enzyme-catalyzed consumption/generation, which are exerted as co-reactants in hole-oxidization reactions and electron moderation, leading to cathodic/anodic photocurrent signal production [143]. Li et al. fabricated a novel sensing stand on the typical catalytic reaction of a hemin/platinum nanoparticle (Pt NP) trunk-branching-decorated DNA dendrimer toward H2O2 [143]. Pt NPs have been widely exerted in bioassays due to their excellent inherited properties involving small size, diverse enzyme-mimic activities, and especially catalytic activity toward H2O2 for electron acceptor (O2) production [145,146]. Thus, in this proposed sensor, the DNA dendrimer serves as enzyme-like activity enhancement and synergy catalysis with Pt NPs, as well as hemin, to catalyze H2O2 and increase electron acceptors; hence, it can determine the target miRNA-141 over a wide range, from 0.5 fM to 5 nM with a limit of detection at 0.17 fM (Figure 6B).
In photoactive material-associated PEC paper-based devices, photoactive species play an essential role serving as a light absorber [119]. In particular, emerging nanozymes are being intensively investigated as an expected alternative to natural enzymes due to their many intrinsic qualities, including superior stability, enzyme-like capabilities, and wide pH operating range [128]. For instance, Li and coworkers fabricated photoelectrochemical paper-based sensing to detect carcinoembryonic antigen (CEA) based on the modification of paper with N-carbon dots/TiO2–Pt in a seed-mediated growth method [144]. First, TiO2 seeds were prepared from a TiCl3 solution in a mixture maintained at 80 °C for 2 h and then dried at 450 °C for 2 h. In the next step, these TiO2 seeds were spread onto cellulose fiber (Pt/PWE) and equilibrated at 37 °C, repeating the process three times. The proposed TiO2 NP-modified photoelectrochemical lab-on-paper analytical devices can determine CEA concentrations based on the reduction of the photoelectrochemical signal, because they bind with CEA at the MCF-7 cell surfaces in human serum (Figure 6C). This novel platform has good biocompatibility, is portable, and has eco-friendly devices that can detect low levels in limitations of 1.0 pg mL–1 over a broad linear range of 0.002–200 ng mL–1. Additionally, Li and colleagues also employed this strategy to develop an ultrasensitive PEC paper-based device to determine miRNA-141 from a two-enzyme-engineered DNA walker and a TiO2/CeO2 heterojunction (Figure 6D) [128]. During cancer development, miRNA-141 is expressed in large proportions, and thus, acts as a potential marker for cancer diagnostics and assessment. In the presence of miRNA-141, the protecting probes would be kept away, leading to activated walker probes, creating an endonuclease cleavage reaction, and eventually, retrieving a signal output. The novel sensor can verify miRNA-141 in real human serum with LOD at 0.6 fM, and can ensure long-term stability and highly satisfactory selectivity.
8. Nucleic Acid-Mediated Signal Amplification
Aptamer-integrated PADs are emerging as potential diagnostic sensors due to the outstanding aptamer advantages of more reasonable costs, high thermo-stability, and non-toxicity, as well as fewer batch-to-batch variations in comparison to antibodies [147,148]. A paper-based nucleic acid amplification test has been developed with the support of nucleic acid amplification and clustered, regularly interspaced, short palindromic repeats (CRISPR)/Cas) systems to improve the sensitivity of PADs.
8.1. Nucleic Acid Amplification
Nucleic acid-associated signal amplification has attracted wide attention in the fabrication of highly sensitive paper-based point-of-care diagnostics employed for invading pathogens or viral determination due to the nucleic acid-amplification capacity. Especially during the current COVID-19 pandemic, the need for an innovative and effective methodology to detect the novel coronavirus (SARS-CoV-2) was evident in order to control, minimize, and finally prevent its spread. Conventionally, polymerase chain reaction (PCR) is the technique extensively used to rapidly process millions to billions of DNA samples, but it requires precise temperature control for reactions, and hence, to date has been unsuccessfully applied to PADs [149,150]. Alternatively, various signal amplification isothermal methods have been tried for PAD development, including rolling circle amplification (RCA) [147,151,152,153,154,155], loop-mediated isothermal amplification (LAMP) [156,157,158,159,160,161,162,163,164,165,166], strand-displacement amplification (SDA) [167], recombinase polymerase amplification (RPA) [168,169,170,171], helicase dependent amplification (HDA) [172,173,174], and nucleic acid sequence-based amplification (NASBA) [175].
The principal difference in the aforementioned isothermal methods stems from primer annealing and extension, and LAMP, RCA, and RPA have been employed widely in PADs. In particular, the LAMP technique provides enlarged specificity and sensitivity based on an exponential amplification capacity that can distinguish primers in one reaction, and hence, can simultaneously identify various target sequences [176]. To determine a mycobacterium’s DNA in a single reaction for the first time, Naik and coworkers successfully designed a novel LAMP assay on a paper substrate to detect E. coli and M. smegmatis (Figure 7A) [161]. This system can perform lysis and amplify DNA from only 100 CFU/mL with high sensitivity within 30 min through recorded fluorescence intensities, especially ensuring safety due to an effective ability to kill all bacteria in the samples. On the other hand, in RCA, this isothermal DNA amplification method can simplify operations, producing 1000 complementary copies in linear concatenated DNA within only 1 h [151]. Bialy et al. constructed a one-pot-reaction paper-based POC device to detect thrombin and platelet-derived growth factor, typically through the suppression of RCA [152]. The results were obtained via the fluorescence signal of SYBR Gold dye and QuantiFluor dye with a very low LOD at 10 nm (3σ) and 25 nm (3σ), respectively, within 30 min at RT. In addition, RPA has been one of the most amplified methods for paper-based nucleic acid biosensor fabrication due to its outstanding ability to rapidly evaluate various gene targets at low temperature with superior primer specificity [170,171]. Based on these advantages, an RPA vertical flow paper microarray was developed for human adenoviral DNA detection by integrating it with colorimetric detection holding antibody-conjugated AuNPs (Figure 7B) [170]. This proposed system enables validation from 1 ng of starting material in clinical nasopharyngeal aspirate samples in less than 10 min for detection, and it is expected to be used for multiplexed viral diagnostics.
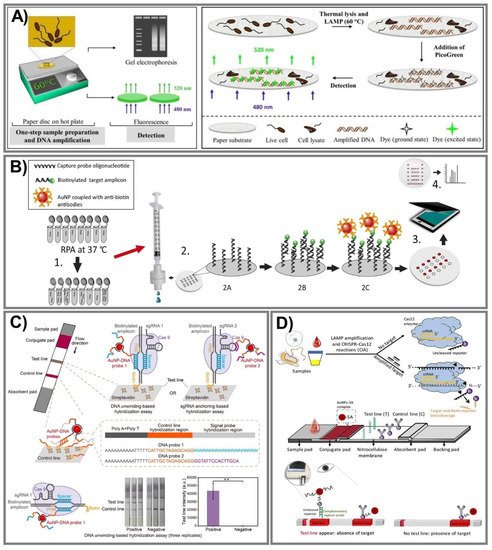
Figure 7. Nucleic acid-amplified PADs. (A) One-step lysis to amplify DNA for E. coli and M. smegmatis in a LAMP system through recorded fluorescence intensities by the binding of DNA with PicoGreen dye. Adapted with permission from Ref. [161]. (B) The process for human adenoviral detection was carried out by novel RPA vertical flow-paper microarray based on the visualization of anti-biotin antibody-conjugated AuNPs with positive spots. Adapted with permission from Ref. [170]. (C) The CRISPR/Cas9-mediated lateral flow nucleic acid assay was established to identify L. monocytogenes and African swine fever virus (ASFV) in swine serum samples. ** p < 0.01 (two-tailed Student’s t test). Adapted with permission from Ref. [177]. (D) An ultrasensitive CRISPR/Cas12 lateral flow sensor was developed by integrating Cas12a and Cas12b effectors to determine Pseudomonas aeruginosa—a multidrug-resistant infection. Adapted with permission from Ref. [178].
8.2. CRISPR/Cas Triggered Signal Amplification
Currently, the CRISPR/Cas effector is attracting a lot of attention because it is not only extensively employed in genome editing, but is also considered an innovative approach in biosensor fabrication. This system can offer much more precise (as well as rapid) analysis for ultra-sensitive nucleic acid evaluation. The CRISPR/Cas9 system has outstanding DNA recognition capacity, but does not provide trans-cleavage activity; hence, even though Cas9 conducted directed evolution with its genome-editing ability, it can still be harnessed to apply to biosensing system construction [177]. Furthermore, Cas12 and Cas13 recently emerged as potential strategies in which Cas12 can create a ternary complex with crRNA (or sgRNA) and a target nucleic acid, while Cas13 can be used against ssRNA, and then labeled with fluorescence or dye to generate fluorescence or colorimetric signals [179]. Various studies have reported excellent capacity in CRISPR/Cas systems, including Cas9 [177,180], Cas12 [178,181], and Cas13 [182]. Wang and coworkers established a CRISPR/Cas9-mediated lateral flow nucleic acid assay (CASLFA) to detect L. monocytogenes and the African swine fever virus, typically in swine serum samples (Figure 7C) [177]. This proposed system can recognize double-stranded DNA (dsDNA) with only hundreds of copies of genome samples in 1 h with an accuracy of 100%, in comparison to a real-time PCR assay. On the other hand, for Cas12 effector applications, an ultrasensitive POC CRISPR/Cas12 lateral flow sensor was developed by integrating Lachnospiraceae bacterium Cas12a and Alicyclobacillus acidoterrestris Cas12b effectors with the LAMP amplification method to determine Pseudomonas aeruginosa—a multidrug-resistant infection (Figure 7D) [178]. The outstanding advantage of this sensor is a non-traditional DNA/RNA extraction requirement when Cas12 serves as a collateral cleavage of ssDNA reporter sequences and cognate targets. Additionally, the excess AuNPs-streptavidin complex conjugating in the proposed platform interacted with biotinylated ssDNA reporters and was then captured by a capture probe to spot the sensing performance. This novel approach is inexpensive, ultrasensitive at the very low concentration of 1 CFU/mL, and can especially discriminate a target from both impure and complex non-target samples.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9050540
This entry is offline, you can click here to edit this entry!
