Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
The MAL gene encodes a 17-kDa protein containing four putative transmembrane segments whose expression is restricted to human T cells, polarized epithelial cells and myelin-forming cells. It organizes condensed membranes to make them functional in specialized pathways of membrane trafficking and cell signaling.
- condensed membranes
- membrane trafficking
- MARVEL domain
1. Introduction
The human MAL cDNA was originally identified during a search for genes that are selectively expressed during T cell differentiation [1]. In addition to T cells, MAL gene expression was detected in specific polarized epithelia and in myelin-forming cells [2][3][4][5]. The MAL gene encodes a highly hydrophobic integral membrane protein of 153 amino acids that is conserved across vertebrates; approximately 53% and 69% of the residues in human MAL are identical or similar, respectively, to those in zebrafish MAL. Structural predictions suggest that MAL has a four transmembrane-helix architecture with its N- and C-terminal ends oriented towards the cytoplasm (Figure 1A) [1].
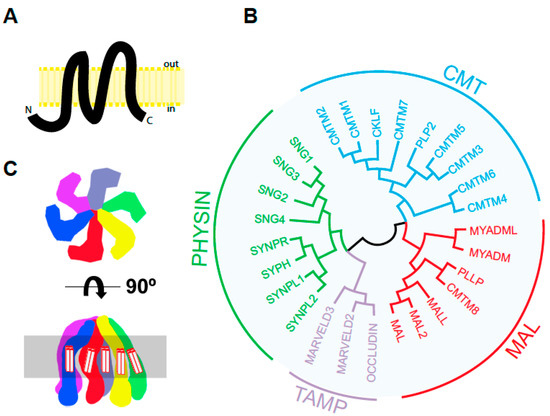
Figure 1. The human MARVEL superfamily of proteins. (A) Predicted structure of human MAL according to Uniprot (https://www.uniprot.org/uniprot/P21145, accessed on 5 April 2021). Protein segments: 1–24 cytoplasmic, 25–46 transmembrane, 47–53 first extracellular loop, 54–75 transmembrane, 76–92 cytoplasmic loop, 93–114 transmembrane, 115–124 second extracellular loop, 126–147 transmembrane, 148–153 cytoplasmic. The length of the different segments in the schematics is drawn to scale. (B) Tree of human MARVEL domain-containing proteins. The sequence of the indicated proteins except their cytoplasmic amino- and carboxyl-terminal tails was aligned using the Muscle algorithm of the Jalview software [6], and the resulting alignment was used in MegaX [7]. The protein accession numbers of the corresponding sequences were: CMTM1 (NP_443725.3), CMTM2 (NP_653274.1), CMTM3 (NP_653201.1), CMTM4 (NP_848933.1), CMTM5 (NP_612469.1), CMTM6 (NP_060271.1), CMTM7 (NP_612419.1), CMTM8 (NP_849199.2), CKLF (NP_058647.1, PLP2/A4 (NP_002659.1), MYADM (NP_612382.1), MYADML2 (NP_001138585.2), MAL (NP_002362.1), MAL2 (NP_443118.1), MALL/BENE (NP_005425.1), PLLP/plasmolipin (NP_057077.1), occludin (NP_002529.1), MARVELD2/tricellulin (NP_001033692.2), MARVELD3 (NP_443090.4), SYPH/synaptophysin (NP_003170.1), SYPL1/pantophysin (NP_006745.1), SYPL2/mitsugumin (NP_001035799.1), SYNPR/synaptoporin (NP_001123475.1), SNG1 (NP_004702.2), SNG2 (NP_004701.1), SNG3 (NP_004200.2) and SNG4 (NP_036583.2). (C) Two views of the three-dimensional structure proposed for the hexameric synaptophysin complex. The highlighted area represents the lipid bilayer. The transmembrane segments of the MARVEL domain are indicated.
MAL shares significant sequence similarity in its four transmembrane domains with other integral membrane proteins. This allows the definition of a domain, called the MARVEL (MAL and related proteins for vesicle formation and membrane link) domain, that is present in 24 human proteins. These constitute the MARVEL superfamily and are conserved across species [8]. The MARVEL superfamily includes the CMTM (chemokine-like factor MARVEL transmembrane domain containing) [9], MAL [10][11], physin [12][13], and TAMP (tight junction-associated MARVEL) [14] families (Figure 1B). The MAL family consists of seven members. MAL2, MALL/BENE, PLLP and CMTM8 display a tetraspanning topology similar to that of MAL. The other two members, myeloid differentiation-associated marker (MYADM) and MYADM-like2 (MYADML2), contain an additional MARVEL domain and form an independent branch within the family. Synaptophysin, which is the most abundant protein in synaptic vesicles [15], is the only MARVEL superfamily member whose 3D structure has been determined so far [16]. Synaptophysin adopts a hexameric basket-like complex with six spokes each, corresponding to a synaptophysin subunit, with an extended, open conformation on the vesicle lumen and a closed end containing the N- and C-termini on the cytosolic side of the vesicle membrane (Figure 1C). This structure is consistent with the proposed role of synaptophysin as a component of the fusion pore during synaptic vesicle exocytosis [17][18]. It is currently unknown whether MAL or other members of the MARVEL superfamily have a similar hexameric structure.
2. The Role of MAL in Epithelial Cells
Polarized epithelial cells transport newly synthesized proteins to the apical surface directly from the Golgi or through an indirect route, which consists of endocytosis of cargo at the basolateral surface and its posterior traffic in vesicular carriers across the cell, in a process known as transcytosis [19][20]. The observations that the apical membrane of epithelial cells is enriched in glycolipids relative to the basolateral surface and that the sorting of glycolipids and proteins takes place at the trans-Golgi network constitute the basis of the proposal of raft-mediated transport to the apical surface [21][22]. This hypothesis was supported by experimental evidence showing that artificial membranes consisting of glycolipids and cholesterol are insoluble in non-anionic detergents such as Triton X-100 [22][23], and by the observation that membrane proteins relying on the direct route, such as the influenza virus hemagglutinin (HA) and GPI-anchored proteins, become resistant to detergent solubilization during biosynthetic transport to the apical membrane [24][25]. In accordance with the original formulation of the raft-mediated model of apical transport [26], the association of glycosphingolipids with cholesterol generates raft platforms at the trans-Golgi network that are immiscible in the other phospholipid-enriched membranes. These platforms recruit specific proteins that are then transported in vesicular carriers made of raft lipids.
2.1. MAL in Apical Transport in Cultured Cells
In the original proposal of rafts as platforms for apical transport, the existence of a specialized protein machinery was postulated to make the rafts competent for transport. This machinery would consist of a minimal set of proteins to ensure the processes of vesicle formation, cargo recruiting, targeting and fusion to the apical surface [26]. One approach used to identify this machinery involved the sequencing of proteins present in detergent-resistant membrane fractions from total cellular membranes after protein separation by two-dimensional gel electrophoresis. Caveolin [27], VIP36 [28], annexin XIIIb [29], and MAL [4] were identified by this approach, and were proposed as candidates to be components of the apical transport machinery. Subsequently, it was shown that exogenous (HA and p75NTR) and endogenous (gp114) single-span transmembrane proteins, chimeric GPI-anchored proteins (YFP-GPI and gD1-DAF), and exogenous (thyroglobulin) and endogenous (gp80/clusterin) secretory proteins [30][31][32][33] require MAL expression to be transported to the apical surface of MDCK cells in an efficient manner. It is of note that one set of studies emphasizes that the kinetics of apical delivery of newly synthesized proteins, but not the targeting of apical proteins, was affected by MAL depletion, as they were found in the apical surface at steady state [31][32][33]. Another study, however, claimed that apical targeting was impaired, as the apical markers assayed concentrated at the Golgi and were nearly absent from the apical surface at steady state [30]. Whether these differences were due to the different systems used to knock down (KD) MAL expression—antisense oligonucleotides in the first case, and inducible expression of antisense RNA in the other—and/or the extent of MAL silencing has not been addressed. The slow kinetics of apical delivery observed in MDCK cells with depleted levels of MAL was recapitulated in epithelial Fisher rat thyroid cells [32], confirming the requirement of MAL for efficient apical transport by the direct route.
Unlike simple epithelial cells that directly transport GPI-anchored and single transmembrane domain proteins from the trans-Golgi network to the apical surface, in hepatocytes these proteins reached the apical membrane by basolateral-to-apical transcytosis [34]. Consistent with the absence of a direct route, MAL is not expressed in hepatocytes and hepatic cell lines such as HepG2 and WIF-B cells [35][36]. It is of note that GPI-anchored proteins and apical single transmembrane proteins do not partition into DRMs in control WIF-B cells but do so in WIF-B cells expressing MAL exogenously, and MAL reroutes them into the direct pathway [36]. These findings support the role of MAL as a component of the machinery of the direct route of apical transport mediated by raft membranes (Figure 2).
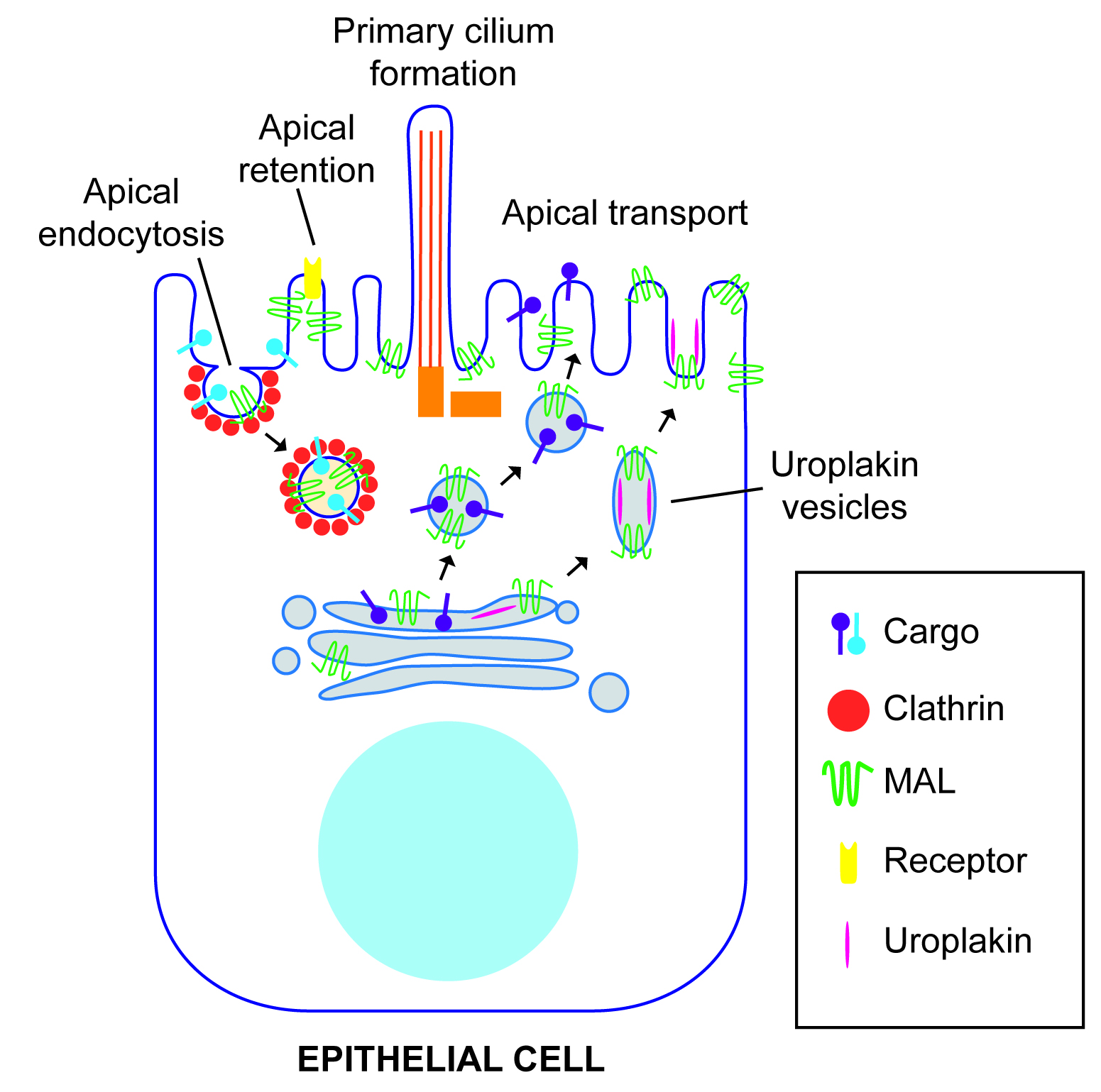
Figure 2. Function of MAL in polarized epithelial cells. MAL has been involved in apical transport of specific cargo from the Golgi. In the case of urothelial cells, the vesicles transport preassembled urothelial plaque units. MAL has also been involved in clathrin-dependent apical endocytosis, and in retention of specific proteins at the apical membrane.
2.2. MAL in Apical Transport: KO Mice
The apical surface of mammalian bladder epithelium contains one of the most effective permeability barriers known to exist in nature, whose function is to protect the epithelium from the urinary components. To accomplish this, the most superficial layer of urothelial cells is almost completely covered by large (0.10–1 μm) two-dimensional crystals of 16-nm uroplakin particles [37], known as urothelial plaques, comprising four major integral membrane proteins called uroplakins: tetraspanins Ia and Ib, and the single-span membrane proteins II and IIIa [38]. The intracellular trafficking of these plaques to the apical membrane is mediated by fusiform vesicles (500–800 nm), which consist of two plaques connected by a flexible, detergent-insoluble hinge region [39][40].
MAL expression is associated with in vitro urothelial cell differentiation [41]. Consistent with this finding, MAL is expressed in human [42] and mouse urothelium [43], as revealed by immunohistochemical analysis. As in other epithelial cells, MAL and uroplakins fractionate into DRMs of urothelial cells. In the superficial layer, MAL is present at the plasma membrane between adjacent plaques, and in the fusiform vesicles exclusively occupies the hinge region, which is the zone involved in fusion with the plasma membrane [43]. Consistent with the role of MAL in apical transport in MDCK cells, MAL knockdown (KD) decreases the rate of delivery, but not the targeting, of exogenous uroplakins to the apical membrane of MDCK cells. It is of note that urothelial cells from MAL knockout (KO) mice accumulate a large number of fusiform vesicles at their apical aspect, although the surface content of urothelial plaques is normal at steady state, indicating that the delivery but not the targeting of uroplakins is affected. In uroplakin-II KO mice, the formation of fusiform vesicles is blocked and, instead, vesicles of smaller size (~200 nm) appear that are positive for MAL but lack uroplakin plaques [43]. These findings suggest that, although MAL and uroplakins are able to form distinct types of vesicles in the absence of the other, they only form fusiform vesicles that are completely functional in plaque delivery when they are expressed together. The fact that MAL is present not only in the hinge areas of the fusiform vesicles but also in the hinge areas of the apical surface suggests that MAL might play a role in the fusion event leading to plaque delivery. This role would be consistent with the presence in MAL of a MARVEL domain, which, in synaptophysin, has been suggested to form part of a fusion pore complex that regulates synaptic vesicle exocytosis [17][18].
Similar to MAL, the v-SNARE protein VAMP8/endobrevin localizes at the hinge zone of fusiform vesicles [44]. Unlike MAL KO mice, which have a normal content of urothelial plaques at their apical surface, superficial urothelial cells from VAMP8 KO mice lack surface urothelial plaques but, as in MAL KO mice, form morphologically normal fusiform vesicles that do not fuse with the apical membrane [44]. Therefore, MAL appears to facilitate SNARE-mediated fusion of fusiform vesicles with the apical surface for efficient urothelial plaque delivery (Figure 2).
2.3. MAL in Apical Endocytosis
Since MAL continuously internalizes from the apical membrane [45], it was of interest know whether its internalization is used for the endocytosis of apical proteins. Consistent with this possibility, MAL and apical polymeric immunoglobulin receptor were visualized in the same endocytic vesicles by videomicroscopy, and were identified in the same clathrin-coated pits and vesicles by electron microscopy. Moreover, MAL silencing experiments indicated that apical, but not basolateral, endocytosis of the receptor requires MAL and clathrin. In contrast, the GPI-anchored folate receptor internalizes from the apical membrane in a MAL and clathrin-independent manner [46]. Therefore, in addition to controlling the traffic of proteins from the Golgi to the apical surface, MAL regulates a specialized pathway of endocytosis from the apical surface (Figure 2).
2.4. MAL in Apical Retention of Membrane Proteins
In the human kidney, MAL is expressed in distal tubules, Henle’s loop and collecting ducts, but not in proximal tubules and glomeruli [42]. The aquaporin-2 (AQP2) water channel is expressed exclusively in epithelial cells of the renal collecting duct [47]. AQP2 regulates water reabsorption by redistributing itself between intracellular vesicles and the apical cell surface in a process controlled by the antidiuretic hormone arginine vasopressin [48]. The renal-specific Na+-K+-2Cl− cotransporter (NKCC2) is expressed in epithelial cells of the ascending limb of Henle’s loop. NKCC2 is responsible for Na+ and Cl− reabsorption and the maintenance of normal blood pressure. Consistent with the absence of MAL in proximal tubules [42], LLC-PK1 cells, which derive from this type of structure, do not express MAL endogenously [49]. Ectopically expressed tagged MAL in LLC-PK1 associates with AQP2 and NKCC2 and causes their retention at the apical surface by decreasing their internalization. This observation was interpreted as indicating that MAL has a role in the apical retention of membrane proteins (Figure 2) [49][50]. However, the overexpression of MAL, the presence of a tag in MAL, and the use of a cell line that does not express endogenous MAL limit the validity of this conclusion. Further work in renal epithelial cells positive for endogenous MAL expression, the analysis of the effect of MAL silencing, and the use of exogenous MAL expression in rescue-of-function experiments are needed to provide more compelling evidence that MAL plays such a role.
2.5. MAL in Apical Lumen Formation
The formation of an apical lumen is a key step during epithelial tissue morphogenesis [51][52]. Lumen-containing organs are spherical, as in the case of the thyroid follicles, or tubular, as in the case of the branched tubules of mammary glands or lungs or the unbranched ones of the sweat glands. Defects in lumen formation are associated with a variety of disorders such as polycystic kidney disease and vascular stenosis, which are characterized by abnormal dilation of renal tubules or a reduction in the lumen size of blood vessels, respectively [53].
Mice overexpressing MAL develop multiple large renal cysts, with a close correlation between MAL expression levels and cyst development, the affected tubular segments expressing the highest levels of MAL. Detailed analyses revealed that the cysts probably developed from the distal convoluted and connecting tubuli, whereas proximal convoluted tubuli appeared to be undamaged [54]. MDCK cells have been widely used as a cell model system to study the process of single lumen formation [19][55]. When these cells are cultured in a three-dimensional matrix such as Matrigel®, they form spheres that progressively mature to form hollow structures, referred to as cysts. These consist of a single layer of epithelial cells with their apical surfaces lining an inner single lumen, and their basolateral surface contacting the extracellular matrix. Defects during this process lead to the formation of multiple lumens of smaller size [53]. Oligonucleotides designed to knock down MAL expression [56], as well as tagged-MAL overexpression [57], produce cysts with multiple lumens in MDCK cells. Since MAL overexpression produces dilated apical surfaces in MDCK cells [30], it is plausible that that the effect of MAL overexpression on the formation of multiple lumens in MDCK cells or of renal cysts in mice arises from the production of a large excess of apical membrane. However, it remains unclear how MAL silencing produces the same effect. In conclusion, although the exact role of MAL in the process is still unknown, fine-tuning MAL levels appears to be important for normal cystogenesis in renal cells.
2.6. MAL and Primary Cilium Biogenesis
The primary cilium is a single appendage that protrudes from the cell surface of most mammalian cells. It is made up of a ciliary membrane that surrounds a microtubule-based scaffold, known as the axoneme, that is derived from the older centriole in the centrosome [58]. Primary cilia recognize a wide range of environmental signals and transmit them to the cell body [59]. Defects in primary cilium functioning are associated with a long list of developmental and degenerative disorders [60][61].
In MDCK cells, MAL accumulates predominantly at the base of the cilium in moderately confluent cultures and also at the primary cilium at high cell density [62]. MAL KD results in a drastic drop in the percentage of ciliated cells [62][56]. The components of the machinery for ciliary growth are recruited normally to the centrosome zone under those conditions but are unable to elongate the primary cilium correctly and, consequently, the remaining cilia are stunted [62]. This effect of MAL silencing seems to be due to deficient condensation of the centrosome-associated membranes needed to build the ciliary membrane [63][64]. The overexpression of tagged MAL in MDCK cells and in transgenic mice also gives rise to fewer, shortened cilia. This may have been caused by excessive condensation of the ciliary membrane precursor associated with the centrosome, although this effect was not investigated at the time. In summary, MAL levels modulate proper primary cilium formation and elongation in renal cells (Figure 2).
This entry is adapted from the peer-reviewed paper 10.3390/cells10051065
References
- Alonso, M.A.; Weissman, S.M. cDNA Cloning and Sequence of MAL, a Hydrophobic Protein Associated with Human T-Cell Differentiation. Proc. Natl. Acad. Sci. USA 1987, 84, 1997–2001.
- Kim, T.; Fiedler, K.; Madison, D.L.; Krueger, W.H.; Pfeiffer, S.E. Cloning and Characterization of MVP17: A Developmentally Regulated Myelin Protein in Oligodendrocytes. J. Neurosci. Res. 1995, 42, 413–422.
- Schaeren-Wiemers, N.; Schaefer, C.; Valenzuela, D.M.; Yancopoulos, G.D.; Schwab, M.E. Identification of New Oligodendrocyte- and Myelin-Specific Genes by a Differential Screening Approach. J. Neurochem. 1995, 65, 10–22.
- Zacchetti, D.; Peranen, J.; Murata, M.; Fiedler, K.; Simons, K. VIP17/MAL, a Proteolipid in Apical Transport Vesicles. FEBS Lett. 1995, 377, 465–469.
- Millán, J.; Puertollano, R.; Fan, L.; Alonso, M.A. Caveolin and MAL, Two Protein Components of Internal Detergent-Insoluble Membranes, Are in Distinct Lipid Microenvironments in MDCK Cells. Biochem. Biophys. Res. Commun. 1997, 233, 707–712.
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191.
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549.
- Sanchez-Pulido, L.; Martin-Belmonte, F.; Valencia, A.; Alonso, M.A. MARVEL: A Conserved Domain Involved in Membrane Apposition Events. Trends Biochem. Sci. 2002, 27, 599–601.
- Duan, H.-J.; Li, X.-Y.; Liu, C.; Deng, X.-L. Chemokine-like Factor-like MARVEL Transmembrane Domain-Containing Family in Autoimmune Diseases. Chin. Med. J. 2020, 133, 951–958.
- Magyar, J.P.; Ebensperger, C.; Schaeren-Wiemers, N.; Suter, U. Myelin and Lymphocyte Protein (MAL/MVP17/VIP17) and Plasmolipin Are Members of an Extended Gene Family. Gene 1997, 189, 269–275.
- Pérez, P.; Puertollano, R.; Alonso, M.A. Structural and Biochemical Similarities Reveal a Family of Proteins Related to the MAL Proteolipid, a Component of Detergent-Insoluble Membrane Microdomains. Biochem. Biophys. Res. Commun. 1997, 232, 618–621.
- Janz, R.; Südhof, T.C.; Hammer, R.E.; Unni, V.; Siegelbaum, S.A.; Bolshakov, V.Y. Essential Roles in Synaptic Plasticity for Synaptogyrin I and Synaptophysin I. Neuron 1999, 24, 687–700.
- Adams, D.J.; Arthur, C.P.; Stowell, M.H.B. Architecture of the Synaptophysin/Synaptobrevin Complex: Structural Evidence for an Entropic Clustering Function at the Synapse. Sci. Rep. 2015, 5, 13659.
- Raleigh, D.R.; Marchiando, A.M.; Zhang, Y.; Shen, L.; Sasaki, H.; Wang, Y.; Long, M.; Turner, J.R. Tight Junction-Associated MARVEL Proteins Marveld3, Tricellulin, and Occludin Have Distinct but Overlapping Functions. Mol. Biol. Cell 2010, 21, 1200–1213.
- Rehm, H.; Wiedenmann, B.; Betz, H. Molecular Characterization of Synaptophysin, a Major Calcium-Binding Protein of the Synaptic Vesicle Membrane. EMBO J. 1986, 5, 535–541.
- Arthur, C.P.; Stowell, M.H.B. Structure of Synaptophysin: A Hexameric MARVEL-Domain Channel Protein. Structure 2007, 15, 707–714.
- Pennuto, M.; Dunlap, D.; Contestabile, A.; Benfenati, F.; Valtorta, F. Fluorescence Resonance Energy Transfer Detection of Synaptophysin I and Vesicle-Associated Membrane Protein 2 Interactions during Exocytosis from Single Live Synapses. Mol. Biol. Cell 2002, 13, 2706–2717.
- Valtorta, F.; Pennuto, M.; Bonanomi, D.; Benfenati, F. Synaptophysin: Leading Actor or Walk-on Role in Synaptic Vesicle Exocytosis? Bioessays 2004, 26, 445–453.
- Rodriguez-Boulan, E.; Kreitzer, G.; Musch, A. Organization of Vesicular Trafficking in Epithelia. Nat. Rev. Mol. Cell. Biol. 2005, 6, 233–247.
- Weisz, O.A.; Rodriguez-Boulan, E. Apical Trafficking in Epithelial Cells: Signals, Clusters and Motors. J. Cell Sci. 2009, 122, 4253–4266.
- van Meer, G.; Stelzer, E.H.; Wijnaendts-van-Resandt, R.W.; Simons, K. Sorting of Sphingolipids in Epithelial (Madin-Darby Canine Kidney) Cells. J. Cell Biol. 1987, 105, 1623–1635.
- Simons, K.; Van Meer, G. Lipid Sorting in Epithelial Cells. Biochemistry 1988, 27, 6197–6202.
- Hagmann, J.; Fishman, P.H. Detergent Extraction of Cholera Toxin and Gangliosides from Cultured Cells and Isolated Membranes. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1982, 720, 181–187.
- Skibbens, J.E.; Roth, M.G.; Matlin, K.S. Differential Extractability of Influenza Virus Hemagglutinin during Intracellular Transport in Polarized Epithelial Cells and Nonpolar Fibroblasts. J. Cell Biol. 1989, 108, 821–832.
- Brown, D.A.; Rose, J.K. Sorting of GPI-Anchored Proteins to Glycolipid-Enriched Membrane Subdomains during Transport to the Apical Cell Surface. Cell 1992, 68, 533–544.
- Simons, K.; Wandinger-Ness, A. Polarized Sorting in Epithelia. Cell 1990, 62, 207–210.
- Kurzchalia, T.V.; Dupree, P.; Parton, R.G.; Kellner, R.; Virta, H.; Lehnert, M.; Simons, K. VIP21, a 21-KD Membrane Protein Is an Integral Component of Trans-Golgi-Network-Derived Transport Vesicles. J. Cell Biol. 1992, 118, 1003–1014.
- Fiedler, K.; Parton, R.G.; Kellner, R.; Etzold, T.; Simons, K. VIP36, a Novel Component of Glycolipid Rafts and Exocytic Carrier Vesicles in Epithelial Cells. EMBO J. 1994, 13, 1729–1740.
- Fiedler, K.; Lafont, F.; Parton, R.G.; Simons, K. Annexin XIIIb: A Novel Epithelial Specific Annexin Is Implicated in Vesicular Traffic to the Apical Plasma Membrane. J. Cell Biol. 1995, 128, 1043–1053.
- Cheong, K.H.; Zacchetti, D.; Schneeberger, E.E.; Simons, K. VIP17/MAL, a Lipid Raft-Associated Protein, Is Involved in Apical Transport in MDCK Cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6241–6248.
- Puertollano, R.; Martin-Belmonte, F.; Millan, J.; de Marco, M.C.; Albar, J.P.; Kremer, L.; Alonso, M.A. The MAL Proteolipid Is Necessary for Normal Apical Transport and Accurate Sorting of the Influenza Virus Hemagglutinin in Madin-Darby Canine Kidney Cells. J. Cell Biol. 1999, 145, 141–151.
- Martin-Belmonte, F.; Puertollano, R.; Millan, J.; Alonso, M.A. The MAL Proteolipid Is Necessary for the Overall Apical Delivery of Membrane Proteins in the Polarized Epithelial Madin-Darby Canine Kidney and Fischer Rat Thyroid Cell Lines. Mol. Biol. Cell 2000, 11, 2033–2045.
- Martin-Belmonte, F.; Arvan, P.; Alonso, M.A. MAL Mediates Apical Transport of Secretory Proteins in Polarized Epithelial Madin-Darby Canine Kidney Cells. J. Biol. Chem. 2001, 276, 49337–49342.
- Mostov, K.E.; Verges, M.; Altschuler, Y. Membrane Traffic in Polarized Epithelial Cells. Curr. Opin. Cell Biol. 2000, 12, 483–490.
- de Marco, M.C.; Martin-Belmonte, F.; Kremer, L.; Albar, J.P.; Correas, I.; Vaerman, J.P.; Marazuela, M.; Byrne, J.A.; Alonso, M.A. MAL2, a Novel Raft Protein of the MAL Family, Is an Essential Component of the Machinery for Transcytosis in Hepatoma HepG2 Cells. J. Cell Biol. 2002, 159, 37–44.
- Ramnarayanan, S.P.; Cheng, C.A.; Bastaki, M.; Tuma, P.L. Exogenous MAL Reroutes Selected Hepatic Apical Proteins into the Direct Pathway in WIF-B Cells. Mol. Biol. Cell 2007, 18, 2707–2715.
- Kachar, B.; Liang, F.; Lins, U.; Ding, M.; Wu, X.-R.; Stoffler, D.; Aebi, U.; Sun, T.-T. Three-Dimensional Analysis of the 16 Nm Urothelial Plaque Particle: Luminal Surface Exposure, Preferential Head-to-Head Interaction, and Hinge Formation 1 1Edited by W. Baumeisser. J. Mol. Biol. 1999, 285, 595–608.
- Wu, X.-R.; Kong, X.-P.; Pellicer, A.; Kreibich, G.; Sun, T.-T. Uroplakins in Urothelial Biology, Function, and Disease. Kidney Int. 2009, 75, 1153–1165.
- Liang, F.; Kachar, B.; Ding, M.; Zhai, Z.; Wu, X.-R.; Sun, T.-T. Urothelial Hinge as a Highly Specialized Membrane: Detergent-Insolubility, Urohingin Association, and in Vitro Formation. Differentiation 1999, 65, 59–69.
- Hudoklin, S.; Jezernik, K.; Neumüller, J.; Pavelka, M.; Romih, R. Electron Tomography of Fusiform Vesicles and Their Organization in Urothelial Cells. PLoS ONE 2012, 7, e32935.
- Liebert, M.; Yuan, T.Y.; Grossman, H.B.; Hubbel, A.; Chung, M.; Wedemeyer, G.; Brozovich, M.; Lomax, M.I.; Hegeman, A.; Wheelock, M.J. Expression of Mal Is Associated with Urothelial Differentiation In Vitro: Identification by Differential Display Reverse-Transcriptase Polymerase Chain Reaction. Differentiation 1997, 61, 177–185.
- Marazuela, M.; Acevedo, A.; Adrados, M.; Garcia-Lopez, M.A.; Alonso, M.A. Expression of MAL, an Integral Protein Component of the Machinery for Raft-Mediated Pical Transport, in Human Epithelia. J. Histochem. Cytochem. 2003, 51, 665–673.
- Zhou, G.; Liang, F.-X.; Romih, R.; Wang, Z.; Liao, Y.; Ghiso, J.; Luque-Garcia, J.L.; Neubert, T.A.; Kreibich, G.; Alonso, M.A.; et al. MAL Facilitates the Incorporation of Exocytic Uroplakin-Delivering Vesicles into the Apical Membrane of Urothelial Umbrella Cells. Mol. Biol. Cell 2012, 23, 1354–1366.
- Wankel, B.; Ouyang, J.; Guo, X.; Hadjiolova, K.; Miller, J.; Liao, Y.; Tham, D.K.L.; Romih, R.; Andrade, L.R.; Gumper, I.; et al. Sequential and Compartmentalized Action of Rabs, SNAREs, and MAL in the Apical Delivery of Fusiform Vesicles in Urothelial Umbrella Cells. Mol. Biol. Cell 2016, 27, 1621–1634.
- Puertollano, R.; Alonso, M.A. MAL, an Integral Element of the Apical Sorting Machinery, Is an Itinerant Protein That Cycles between the Trans-Golgi Network and the Plasma Membrane. Mol. Biol. Cell 1999, 10, 3435–3447.
- Martin-Belmonte, F.; Martinez-Menarguez, J.A.; Aranda, J.F.; Ballesta, J.; de Marco, M.C.; Alonso, M.A. MAL Regulates Clathrin-Mediated Endocytosis at the Apical Surface of Madin-Darby Canine Kidney Cells. J. Cell Biol. 2003, 163, 155–164.
- Nielsen, S.; Frøkiær, J.; Marples, D.; Kwon, T.-H.; Agre, P.; Knepper, M.A. Aquaporins in the Kidney: From Molecules to Medicine. Physiol. Rev. 2002, 82, 205–244.
- Nielsen, S.; Kwon, T.-H.; Frøkiær, J.; Agre, P. Regulation and Dysregulation of Aquaporins in Water Balance Disorders. J. Intern. Med. 2007, 261, 53–64.
- Kamsteeg, E.-J.; Duffield, A.S.; Konings, I.B.M.; Spencer, J.; Pagel, P.; Deen, P.M.T.; Caplan, M.J. MAL Decreases the Internalization of the Aquaporin-2 Water Channel. Proc. Natl. Acad. Sci. USA 2007, 104, 16696–16701.
- Carmosino, M.; Rizzo, F.; Procino, G.; Basco, D.; Valenti, G.; Forbush, B.; Schaeren-Wiemers, N.; Caplan, M.J.; Svelto, M. MAL/VIP17, a New Player in the Regulation of NKCC2 in the Kidney. Mol. Biol. Cell 2010, 21, 3985–3997.
- Bryant, D.M.; Roignot, J.; Datta, A.; Overeem, A.W.; Kim, M.; Yu, W.; Peng, X.; Eastburn, D.J.; Ewald, A.J.; Werb, Z.; et al. A Molecular Switch for the Orientation of Epithelial Cell Polarization. Dev. Cell 2014, 31, 171–187.
- Rodriguez-Boulan, E.; Macara, I.G. Organization and Execution of the Epithelial Polarity Programme. Nat. Rev. Mol. Cell. Biol. 2014, 15, 225–242.
- Datta, A.; Bryant, D.M.; Mostov, K.E. Molecular Regulation of Lumen Morphogenesis. Curr. Biol. 2011, 21, R126–R136.
- Frank, M.; Atanasoski, S.; Sancho, S.; Magyar, J.P.; Rülicke, T.; Schwab, M.E.; Suter, U. Progressive Segregation of Unmyelinated Axons in Peripheral Nerves, Myelin Alterations in the CNS, and Cyst Formation in the Kidneys of Myelin and Lymphocyte Protein-Overexpressing Mice. J. Neurochem. 2000, 75, 1927–1939.
- Wang, A.Z.; Ojakian, G.K.; Nelson, W.J. Steps in the Morphogenesis of a Polarized Epithelium. I. Uncoupling the Roles of Cell-Cell and Cell-Substratum Contact in Establishing Plasma Membrane Polarity in Multicellular Epithelial (MDCK) Cysts. J. Cell Sci. 1990, 95, 137–151.
- Torkko, J.M.; Manninen, A.; Schuck, S.; Simons, K. Depletion of Apical Transport Proteins Perturbs Epithelial Cyst Formation and Ciliogenesis. J. Cell Sci. 2008, 121, 1193–1203.
- Takiar, V.; Mistry, K.; Carmosino, M.; Schaeren-Wiemers, N.; Caplan, M.J. VIP17/MAL Expression Modulates Epithelial Cyst Formation and Ciliogenesis. Am. J. Physiol. Cell Physiol. 2012, 303, C862–C871.
- Bernabe-Rubio, M.; Alonso, M.A. Routes and Machinery of Primary Cilium Biogenesis. Cell. Mol. Life Sci. 2017, 74, 4077–4095.
- Nachury, M.V.; Mick, D.U. Establishing and Regulating the Composition of Cilia for Signal Transduction. Nat. Rev. Mol. Cell. Biol. 2019, 20, 389–405.
- Braun, D.A.; Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028191.
- Reiter, J.F.; Leroux, M.R. Genes and Molecular Pathways Underpinning Ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547.
- Reales, E.; Bernabé-Rubio, M.; Casares-Arias, J.; Rentero, C.; Fernández-Barrera, J.; Rangel, L.; Correas, I.; Enrich, C.; Andrés, G.; Alonso, M.A. The MAL Protein Is Crucial for Proper Membrane Condensation at the Ciliary Base, Which Is Required for Primary Cilium Elongation. J. Cell Sci. 2015, 128, 2261–2270.
- Bernabé-Rubio, M.; Bosch-Fortea, M.; García, E.; de la Serna, J.B.; Alonso, M.A. Adaptive Lipid Immiscibility and Membrane Remodeling Are Active Functional Determinants of Primary Ciliogenesis. Small Methods 2020, 5, 2000711.
- Labat-de-Hoz, L.; Rubio-Ramos, A.; Casares-Arias, J.; Bernabé-Rubio, M.; Correas, I.; Alonso, M.A. A Model for Primary Cilium Biogenesis by Polarized Epithelial Cells: Role of the Midbody Remnant and Associated Specialized Membranes. Front. Cell Dev. Biol. 2021, 8, 622918.
This entry is offline, you can click here to edit this entry!
