Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Telomerase reverse transcriptase (TERT) mutations are reportedly the most frequent somatic genetic alterations in hepatocellular carcinoma (HCC).
- telomere
- TERT
- liver neoplasm
- biomarkers
- treatment outcome
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fifth leading cause of cancer-related mortality worldwide [1]. The major risk factors for HCC include liver cirrhosis, hepatitis B or C virus infection, alcohol abuse, nonalcoholic steatohepatitis, and metabolic disease. The mutation landscape in liver carcinogenesis is reportedly complicated, involving a number of pathways as well as somatic mutations in a multitude of genes [2][3][4]. Among the genetic alterations, telomerase reverse transcriptase (TERT) promoter mutations were reported to occur early and most frequently, affecting approximately 30–60% of all HCC patients [5][6]. Two hotspot mutations at −124 and −146 positions from the ATG start site in the TERT promoter have been shown to regulate TERT expression or the telomerase activation of human malignancies, including HCC [6][7].
Telomeres, repetitive DNA sequences (TTAGGG in vertebrates) found at the ends of the chromosome, are gradually shortened by each cell division in somatic cells, finally reaching senescence or apoptosis [8][9]. Telomeres are elongated by telomerase, a ribonucleoprotein–reverse transcriptase complex that uses its RNA as a template for the addition of simple telomeric repeats. Telomerase activity is regulated by the telomere-binding protein complex, called shelterin, which is composed of six proteins, including the telomeric repeat-binding factors (TRF) 1 and TRF2, the TRF1-interacting protein 2 (TIN2), protection of telomeres 1 (POT1), the POT1–TIN2 organizing protein (TPP1), and repressor/activator protein 1 (RAP1) [8]. However, embryonic stem cells and most cancer cells can maintain telomeres to overcome cell senescence or apoptosis. This process is controlled by TERT, the catalytic component of the telomerase complex that maintains telomere ends by addition of the telomere repeat TTAGGG [4][9]. Thus, TERT plays an important role in oncogenesis and the immortality of cancer cells.
Abnormalities in TERT expression or promoter mutations in HCC have been sporadically studied and reported to be associated with cancer recurrence and progression [10][11][12][13][14]. However, the clinical implications and molecular mechanisms underlying HCC initiation and progression are still unclear because only limited studies have been conducted and the previous studies included only a small number of patients or were limited only to the surgical setting of early-stage HCC. Thus, there is insufficient knowledge about how and what changes in TERT occur from early- to late-stage HCC. Moreover, no studies have attempted to evaluate the impact of the TERT-telomere network and alterations on the outcomes of various stages of HCC treated by surgical and non-surgical options.
To address these issues, the present study correlated the genetic alterations in TERT, gene expression, and telomere length with the clinicopathological features of HCC. In addition, the potential roles of the TERT-telomere network as a prognostic biomarker within the setting of hepatectomy and transarterial chemoembolization (TACE) were evaluated and compared.
2. Protein–Protein Interaction with TERT Gene Sets and Gene Expression
The protein–protein interaction (PPI) analysis was performed through CBS probe PINGSTM based on the STRING database to establish a set of genes interacting with TERT. Functional clustering of the PPI analysis identified eight genes, including TERT, AKT1, CCT5, mTOR, RPS6KB1, TUBA1B, YWHAQ, and YWHAZ, as protein complexes related to TERT (Figure 1A). Functional interactions between the eight proteins are summarized in Table S1. Within the eight genes, we performed gene expression analysis in the tumor and non-tumor paired samples. TERT mRNA was significantly overexpressed in the tumors compared to the non-tumors (p < 0.001), whereas the expression levels of the other seven genes were not significantly different between the tumors and non-tumors (Figure 1B).
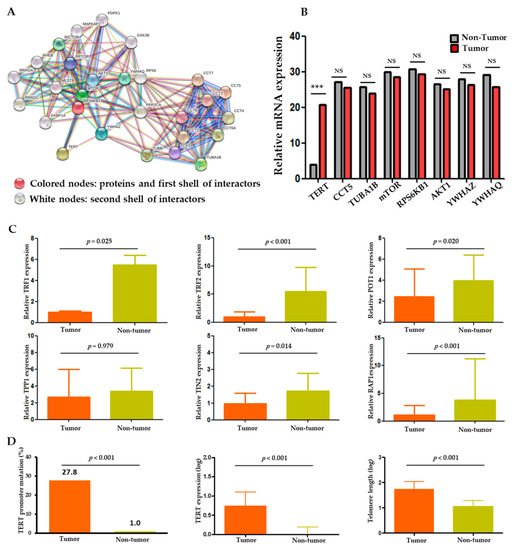
Figure 1. TERT expression and telomere length relationship in HCC. (A) Protein–protein interaction (PPI) analysis of TERT using the CBS probe PINGSTM in HCC tissues. (B) Expression profiles of the eight TERT-interacting genes from the STRING database in tumor versus adjacent non-tumor tissues. (C) Comparison of shelterin complex TRF1, TRF2, POT1, TPP1, TINP2, and RAP1 between tumor and non-tumor tissues. (D) Comparison of TERT promoter mutations, TERT expression and telomere length between tumor and non-tumor tissues. TRF, telomeric repeat-binding factors; POT1, protection of telomeres 1; TPP1, POT1-TIN2 organizing protein; TIN2, TRF1 and TRF2 interacting nuclear protein 2; RAP1, repressor/activator protein 1. NS, non-significance; *** p < 0.001.
Besides the genes derived from the PPI analysis, we also analyzed the shelterin complex which is known to regulate telomerase activity [8]. As a result, all shelterin components (TRF1, TRF2, POT1, TIN2, and RAP1) except TPP1 were significantly overexpressed in the non-tumors compared to the tumors (p < 0.001; Figure 1C). Negative correlations were seen between the expression statuses of the shelterin complex proteins, TERT, and telomere length (Table S2 and Figure S1).
3. Comparison of TERT and Telomere Length in Tumors versus Non-Tumors
Overall, the TERT gene and its function were markedly altered in the tumors compared to the adjacent non-tumor samples. Two hotspot mutations in the TERT promoter region, −124 C>T (C228T) and −146 C>T (C250T), were observed in 57 (27.8%) HCC samples but in only one (1.0%) non-tumor sample (p < 0.001; Figure 1D). Telomere lengths were assessed in 86 evaluable samples. The absolute telomere length was 39.8 ± 2.9 kb (interquartile range: 10.79–110.41), with 55.9 ± 4.1 kb for the tumor and 8.8 ± 0.6 kb for the non-tumor samples. The TERT expression and telomere length were significantly higher and longer, respectively, in the tumors than in the adjacent non-tumor tissues (all p < 0.001; Figure 1D). These findings indicate an important role of TERT and telomere dysfunction in hepatocarcinogenesis.
4. Alterations in TERT and Telomere Length in Relation to Clinical and Tumor Characteristics
The 57 tumoral TERT promoter mutations included 54 (94.7%) with −124 C>T and 3 (5.3%) with −146 C>T mutations. These two mutations were mutually exclusive in HCC. The prevalence of TERT promoter mutations differed according to the etiology of liver disease. The 57 patients with the mutation consisted of 32 with HBV-related HCC (23.2%), 7 with HCV-related HCC (43.8%), and 18 with NBNC (non-HBV and non-HCV) (35.3%), showing that the mutation rates were highest in HCV-related HCCs and lowest in HBV-related HCCs (Figure 2A). In the tumors, the presence of TERT promoter mutation may be associated with an increasing level of TERT expression and telomere length (Figure 2B), which suggests that tumoral TERT expression and telomere length were affected by the TERT promoter mutation status.
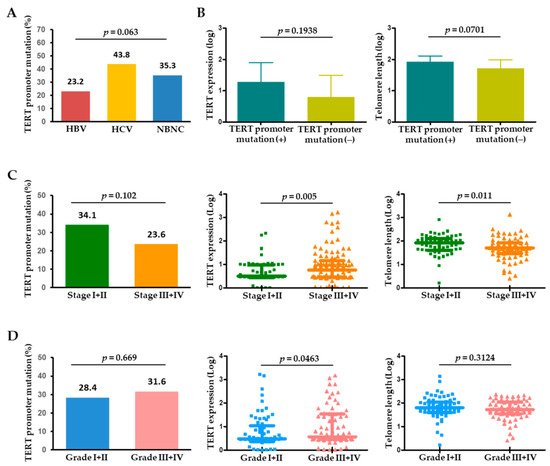
Figure 2. (A) Frequency of TERT promoter mutations in HCC according to the etiology of HCC. (B) TERT expression and telomere length in the presence or absence of TERT promoter mutations in HCC. Frequency of TERT promoter mutations, TERT expression, and telomere length according to (C) tumor stage and (D) tumor histological grade. HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-HBV non-HCV.
When analyzed by tumor characteristics, TERT promoter mutations tended to be more frequent in single (37/109, 33.9%) versus multiple (20/92, 21.7%) HCC (p = 0.056). With tumor stage progression, the TERT level was significantly upregulated (p = 0.005), whereas telomere length significantly decreased (p = 0.011) (Figure 2C). Regarding pathological tumor differentiation, TERT expression levels were positively correlated with HCC histological grade, while TERT promoter mutations or telomere length were not significantly different between the grades (Figure 2D).
This entry is adapted from the peer-reviewed paper 10.3390/cancers13092160
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
- Lee, J.-S. The mutational landscape of hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 220.
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.-C.; Llovet, J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015, 149, 1226–1239.e1224.
- Ma, Z.-X.; Yang, C.-M.; Li, M.-G.; Tu, H. Telomerase reverse transcriptase promoter mutations in hepatocellular carcinogenesis. Hepatoma Res. 2019, 2019.
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 1–7.
- Nault, J.-C.; Ningarhari, M.; Rebouissou, S.; Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 15, 544–558.
- Huang, D.-S.; Wang, Z.; He, X.-J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.-Y.; Wang, W.; Jiang, X.-T. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976.
- Okamoto, K.; Seimiya, H. Revisiting telomere shortening in cancer. Cells 2019, 8, 107.
- In der Stroth, L.; Tharehalli, U.; Günes, C.; Lechel, A. Telomeres and Telomerase in the Development of Liver Cancer. Cancers 2020, 12, 2048.
- Kawai-Kitahata, F.; Asahina, Y.; Tanaka, S.; Kakinuma, S.; Murakawa, M.; Nitta, S.; Watanabe, T.; Otani, S.; Taniguchi, M.; Goto, F. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J. Gastroenterol. 2016, 51, 473–486.
- Lee, H.W.; Park, T.I.; Jang, S.Y.; Park, S.Y.; Park, W.-J.; Jung, S.-J.; Lee, J.-H. Clinicopathological characteristics of TERT promoter mutation and telomere length in hepatocellular carcinoma. Medicine 2017, 96, e5766.
- Yu, J.I.; Choi, C.; Ha, S.Y.; Park, C.-K.; Kang, S.Y.; Joh, J.-W.; Paik, S.W.; Kim, S.; Kim, M.; Jung, S.H. Clinical importance of TERT overexpression in hepatocellular carcinoma treated with curative surgical resection in HBV endemic area. Sci. Rep. 2017, 7, 1–12.
- Ako, S.; Nouso, K.; Kinugasa, H.; Matsushita, H.; Terasawa, H.; Adachi, T.; Wada, N.; Takeuchi, Y.; Mandai, M.; Onishi, H.; et al. Human Telomerase Reverse Transcriptase Gene Promoter Mutation in Serum of Patients with Hepatocellular Carcinoma. Oncology 2020, 98, 311–317.
- Li, X.; Xu, W.; Kang, W.; Wong, S.H.; Wang, M.; Zhou, Y.; Fang, X.; Zhang, X.; Yang, H.; Wong, C.H. Genomic analysis of liver cancer unveils novel driver genes and distinct prognostic features. Theranostics 2018, 8, 1740.
This entry is offline, you can click here to edit this entry!
