Vitamin C is an indispensable micronutrient in the human diet due to the multiple functions it carries out in the body. Reports of clinical studies have indicated that, when administered at high dosage by the intravenous route, vitamin C may exert beneficial antitumor effects in patients with advanced stage cancers, including those refractory to previous treatment with chemotherapy.
- vitamin C
- ascorbate
- TET2
- 5-hydroxymethylcytosine
- PARP inhibitors
- IDH1/2
- WT1
- hypomethylating agents
- ROS
- DNA damage
1. Introduction
Vitamin C is an indispensable micronutrient in the human diet due to the multiple functions it carries out in the body. In fact, humans are one of the few species unable to synthesize vitamin C through the oxidation of glucose due to the lack of gulonolactone (L-) oxidase. The importance of vitamin C in human diet was discovered in the 17th century, when the British Royal Navy surgeon James Lind managed to reduce the onset of scurvy (a disease consequent to a prolonged vitamin C deficiency) by introducing citrus fruits, such as lemons and oranges, into the diet of British navy sailors [1]. It was around the 1930s that Albert Szent-Györgyi (Nobel Prize for Medicine in 1937) and Charles Glen King isolated vitamin C [2]. This vitamin participates as co-factor to the hydroxylation of proline and lysine residues of type 1 collagen; thus, it is essential for the formation of collagen and helps maintaining the integrity of the connective and bone tissues, as well as tooth dentin [3]. When vitamin C intake is below 10 mg/d for long periods (>1 month), failure of wound healing, small hemorrhages, bleeding gums, keratosis pilaris, and other systemic and dermatological conditions occur [4][5][6][7][8].
Starting in the second half of the twentieth century, the pioneering studies of Pauling and Cameron proposed vitamin C as a useful agent for the prevention and treatment of cancer [9][10][11]. In these studies, vitamin C was administered first intravenously (I.V.) and then orally as maintenance therapy. Conversely, Moertel and colleagues, showed no positive effects of orally administered vitamin C in cancer patients [12][13]. The debate on vitamin C antitumor efficacy is still ongoing and a number of studies are attempting to establish its role in the treatment of cancer [14]. In this context, particularly interesting are the results of a high number of in vitro studies showing that vitamin C exerts cytotoxic activity on cancer cells, while it is devoid of toxic effects toward normal cells. A number of issues, including the complexity of the biochemical mechanisms regulated by vitamin C and the different experimental models utilized to evaluate its antitumor activity, make it difficult to draw unequivocal conclusions from the preclinical studies carried out over the years. Furthermore, the clinical studies performed in cancer patients in the last decades have shown vitamin C efficacy only in some cases, but biomarkers for predicting patients’ response have not been identified, yet [11][13][15].
2. Chemical Forms of Vitamin C
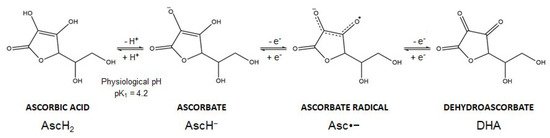
Vitamin C is the common name of L-ascorbic acid, which can be found in different chemical forms. Ascorbic acid (AscH2) (a Latin-derived word, meaning “without scurvy”) is highly soluble in water, but the presence of two ionizable hydroxyl groups makes the compound pH sensitive (pK1 = 4.2; pK2 = 11.6). In biological systems, ascorbic acid loses a proton forming the ascorbate anion (AscH−), the reduced dominant form of vitamin C at physiological pH. Thereafter, AscH− undergoes oxidation and this reaction is dependent on pH and is accelerated by catalytic metals (e.g., iron). In particular, AscH− undergoes one-electron oxidation to form the ascorbate radical (Asc•−). Donation of the second electron gives rise to dehydroascorbic acid (DHA), the fully oxidized form of vitamin C (Figure 2). The ascorbate radical is relatively unreactive since two ascorbate radical anions (Asc•−) can form a dimer and further undergo a disproportionation reaction to form DHA and AscH− [16][17]. These oxidation reactions are coupled with reactive oxygen species (ROS) formation and metals reduction. Spontaneous autoxidation may also occur, but at pH 7 this reaction is very slow.
After transporter-mediated entry into the cell, the DHA form is reduced back to ascorbate. This reaction involves glutathione (GSH) and enzymatic activities like glutaredoxin or other dehydroascorbate reductases with generation of the oxidized glutathione disulfide form (GSSG). The subsequent reduction in GSSG is mediated by the NADPH-dependent glutathione reductase [18]. Thus, cells like erythrocytes or astrocytes may efficiently recycle extracellular DHA by taking it up through GLUT transporters (see also below) [19]. This process allows the recycling and reuse of ascorbate for cellular processes, allowing the antioxidant activity of vitamin C to occur.
Ascorbate also donates electrons to metals such as copper and iron, regulating the activity of enzymes belonging to the families of copper-containing monooxygenases and Fe2+-dependent and α-ketoglutarate-dependent dioxygenases (αKGDD). The first family includes the dopamine β-monooxygenase, necessary for the synthesis of norepinephrine, and the peptidylglycine α-amidating monooxygenase, responsible for a post-translational modification required for the full activation and stabilization of bioactive peptides (e.g., neuroendocrine peptides) [20]. The second and larger family includes several hydroxylases involved in various functions such as type 1 collagen synthesis, carnitine synthesis, tyrosine catabolism, stability of hypoxia-inducible factor α (HIF-1α) and epigenetic modifications [20][21][22][23]. The ability to donate one or two electrons makes ascorbate an excellent reducing agent and antioxidant system in humans [20][24][25][26].
3. Vitamin C Transport into the Cells
In humans, vitamin C is obtained from the diet that contains both ascorbate and DHA. Ascorbate entry into cells is mediated by sodium-dependent vitamin C transporters (SVCT), which comprise two isoforms SVCT1 and SVCT2 (encoded by the SoLute Carrier family 23 member 1 and 2 genes, SLC23A1 and SLC23A2, respectively) that actively co-transport sodium and ascorbate [17][27]. These transporters bind first one molecule of Na+, then one molecule of ascorbate, and finally an additional Na+ [28]. Ascorbate entry into tissues from blood vessels is mediated by a para-cellular movement of ascorbate through gaps between endothelial cells, even though these cells express a large number of SVCT2 [27][29]. SVCT1 is expressed at the apical level in the epithelial intestinal and renal proximal tubules cells in addition to liver and lung [30][31][32]. SVCT1 is involved in the intestinal absorption of vitamin C and in its renal re-absorption back to the blood [33]. On the other hand, SVCT2 is expressed throughout the body tissues (with the exception of red blood cells), including the basolateral side of intestinal epithelial cells [17][31][34][35][36]. The uptake of ascorbate is very tightly controlled. In fact, these transporters are sensitive to intracellular ascorbate concentrations, being upregulated or downregulated in the presence of low or high ascorbate levels, respectively. Therefore, this uptake-pathway maintains the homeostatic physiological concentration of vitamin C in the blood [37][38]. When Ca2+ and Mg2+ are absent, the SVCT2 transport system is in an inactive conformation, despite the presence of Na+ [28].
Another possibility for vitamin C entry into the cells is in its DHA form that is transported through a facilitated diffusion mechanism by glucose transporters (GLUT) that belong to the SLC2 family. GLUT2 and GLUT8 (encoded by the SLC2A2 and SLC2A8 genes, respectively) are expressed only in the intestine and are used by DHA to enter into the enterocytes [39]. Although, a diet rich in free sugars inhibits DHA gut absorption, complex carbohydrates do not affect DHA transport due to the glucose release in jejunum [39]. In this way, DHA can be absorbed in the duodenum by GLUT2 and GLUT8 [39]. In all other human tissues, DHA competes with glucose to be transported via GLUT1 and GLUT3 isoforms (encoded by the SLC2A1 and SLC2A3 genes, respectively) [40].
The GLUTs affinity for DHA (Km ~1–3 mM) is lower than SVCTs affinity for ascorbate (Km ~20–100 μM) [17][36][41][42]. In normal conditions, the glucose concentration (2–5 mM) in the blood is much higher than DHA (˂2 μM); therefore, cell uptake of ascorbate through SVCT2 is preferred [17][30]. Moreover, cells may change their transporter expression depending on vitamin C plasma and intracellular concentrations [17]. Glucose blood concentration and receptor affinity markedly influence the uptake of DHA. Interestingly, the rate of DHA uptake via GLUT1 and GLUT3 in cancer cells is faster than the uptake of ascorbate through SVCT2, even in the presence of glucose [27][43]. In tumor microenvironment oxidizing conditions, the prominent extracellular form of vitamin C is likely DHA that is taken up by the cells and rapidly reduced back to ascorbate, creating a steep gradient across the cell membrane. Furthermore, due to the high requirement of glucose by cancer cells for their metabolism, the GLUT transporters are up-regulated, contributing to DHA intake [44][45][46][47].
4. Vitamin C Dose-Dependent Pharmacokinetics
The main factors that affect the bioavailability of vitamin C are the absorption rate at the intestinal level (in the case of oral formulations or dietary intake) and renal re-absorption. Vitamin C plasma levels show a dose-dependent pharmacokinetics and a first order kinetics of elimination [48][49][50][51]. Studies in humans have revealed that oral doses exceeding 250 mg/day produce plateau plasma concentrations that never exceed 100 µM [4][52]. Phase 1 studies identified 3 g of vitamin C orally administered every 4 h (12 g/day) as the maximum tolerated dose, with maximum plasma concentrations of 220 µM [53]. When plasma ascorbate levels are lower than physiological plasma concentrations (oral doses 0.1 g/d) (i.e., in deficient intake periods), the kidney actively re-absorbs ascorbate back into the bloodstream, preventing the occurrence of acute scurvy [52]. In this condition, the half-life of plasma ascorbate is long (days). In contrast, when the plasma ascorbate levels are higher than 70–80 µM (oral doses >100 g/d), renal excretion increases because of saturated tubular re-absorption at the kidney level and the ascorbate plasma half-life is very short (~30 min) [48][54][55][56].
On the contrary, studies performed in cancer patient have demonstrated that after I.V. administration, the tight control mechanisms of intestinal absorption are by-passed and the observed vitamin C plasma concentrations are in the millimolar range (around 100-fold higher than those detected after oral doses). In particular, vitamin C reaches a plasma peak higher than 20 mM and shows a half-life of 2 h (1.7 h–2.5 h) [50][57]. Moreover, the elevated inflammation and oxidative stress presents in cancer patients results in increased vitamin C utilization and lower plasma levels comparing to healthy people [58][59][60]. Overall, since vitamin C pharmacokinetic properties depend on the route of administration used, the results of studies with oral or intravenous doses are not directly comparable [53].
5. Mechanisms of Vitamin C Anticancer Action
The antitumor effects of vitamin C have been consistently demonstrated by using in vitro cultures of cancer cell lines of different tissue origin (e.g., ovarian, pancreatic adenocarcinoma, lymphoma) and in vivo murine models [61][62][63][64][65][66][67][68][69][70].
5.1. Vitamin C as Pro-Oxidant Agent
The first mechanism described to explain vitamin C antitumor activity relies on the pro-oxidant effects observed following high-dose administration. The pro-oxidant properties have been attributed to the ability of ascorbate to reduce Fe3+ to Fe2+ with consequent generation of ROS through the Fenton reaction (Figure 3). In fact, tumor cells contain higher levels of labile iron (Fe2+) compared to normal cells and this favors higher ROS generation [71]. Moreover, when administered at high dosage, ascorbate may induce the release of Fe2+ from storage proteins [72]. Vitamin C-induced ROS production is further potentiated by the presence of O2 [73].
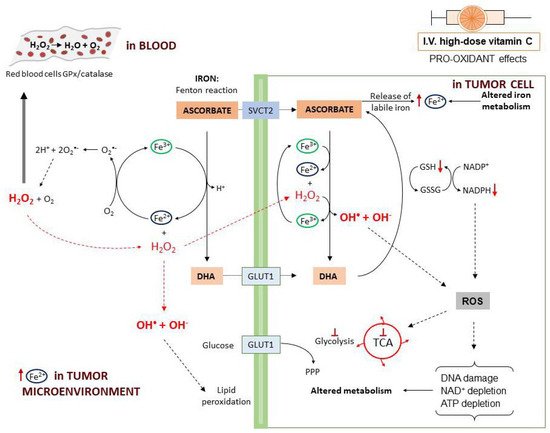
In particular, a study on human Burkitt’s lymphoma cells reported that, at 2 mM concentration, vitamin C is oxidized to the ascorbate radical with production of H2O2 [61]. In a rat model, it was found that parenteral administration of vitamin C induced H2O2 production depending on the dose. Furthermore, high-dose ascorbate may induce H2O2 formation through induction of members of the NADPH oxidase family (DUOX1 and 2) [74]. When plasma ascorbate levels are higher than 1 mM, in the interstitial (extracellular) fluids the ascorbate radical concentration exceeds 100 nM [62][63]. In the blood, H2O2 is rapidly reduced back to H2O by the glutathione peroxidase (GPx) and catalase reduction systems on the erythrocytes plasma membrane. In this way, H2O2 is undetectable in the blood [61][62]. On the contrary, in the extracellular matrix, H2O2 undergoes accumulation and, in the tumor microenvironment, by interaction with Fe2+ can generate hydroxyl radicals that induce cell damage externally, through membrane lipid peroxidation [61][62]. After entering into the cells, H2O2 reacts with intracellular Fe2+ leading to a continuous production of highly damaging hydroxyl radicals. This ROS accumulation directly damages mitochondria and DNA with consequent poly(ADP-ribose) polymerase (PARP) overactivation and NAD+ depletion [75]. In the presence of DHA, the consumption of NADPH used to generate GSH from GSSG eventually results in blockade of glycolysis [46][76]. Ascorbate also induces metabolic shift toward pentose phosphate pathway (PPP), glycerol synthesis and disruption of the tricarboxylic acid cycle (TCA) [77]. Moreover, ROS production leads to inhibition of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and this contributes to glycolytic blockage. In a KRAS/BRAF mutated colorectal cancer cell model, a reversible oxidation of GAPDH was observed after vitamin C treatment [46]. On the other hand, in a neuroblastoma cell line, vitamin C-induced cell death was prevented by the addition of NAD+, demonstrating that GAPDH was inhibited as a consequence of NAD+ depletion [69]. These effects cumulatively lead to ATP depletion and cell death [46][64][77][78][79].
Depending on their levels, ROS have been shown to exert both beneficial and deleterious effects [80]. It is recognized that, compared to normal cells, cancer cells have a greater amount of basal intracellular ROS that promote tumor progression [81][82][83][84]. Tumor cells can tolerate elevated ROS levels, which derive, at least in part, from the glucose-dependent cancer cell metabolism (Warburg effect), by increasing the expression of GSH system. High-dose vitamin C may kill tumor cells either because it alters important cell signaling pathways regulated by ROS (e.g., cell proliferation, migration, neovessel formation) or because it further increases ROS levels causing cellular damage beyond the available defenses systems [85]. Moreover, vitamin C may decrease the antioxidant cellular defenses by selectively reducing the GSH content in tumor cells and not in normal cells [86][87][88]. These aspects have been thoroughly discussed in a recently published review [18].
5.2. Vitamin C as Enzymatic Regulator of TET Enzymes
Vitamin C has recently been found to be an epigenetic modulator through its effects on Ten Eleven Translocation (TET) enzymes, a family of αKGDD enzymes involved in active DNA demethylation (direct removal of a methyl group independently of DNA replication) [89][90]. TET enzymes catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-carboxylcytosine (5fC) and 5-formylcytosine (5cC), followed by conversion to cytosine by the base excision repair (BER). An altered regulation of these enzymes is implicated in tumor development and maintenance. TET mutations (mostly TET2) result in nonfunctional forms of the enzyme, leading to gene promoter hypermethylation. Vitamin C acts as a co-factor for TET enzymes through a direct interaction with their C-terminal catalytic domain and, to a lesser extent, by reducing Fe3+ to Fe2+, making the latter available for TET activity [90][91]. TET function and intracellular vitamin C are both involved in reprogramming and maintaining self-renewal of stem cells [92][93][94].
The three TET1, TET2, and TET3 members have a different tissue distribution and appear to be altered in certain tumors. In particular, TET2 is frequently mutated in both myeloid and lymphoid hematological malignancies and restoration of TET2 blocks aberrant self-renewal of pre-leukemic stem cells [95][96][97][98]. Consistently, in acute myeloid leukemia (AML) cells with TET2 mutations, treatment with vitamin C mimicked TET2 restoration by increasing TET activity (Figure 4) and blocked leukemia progression in patient-derived tumor xenograft models [98]. However, the ability of vitamin C to restore TET2 activity seems to depend on N- and C-terminal lysine acetylation and type of TET2 mutations [99].
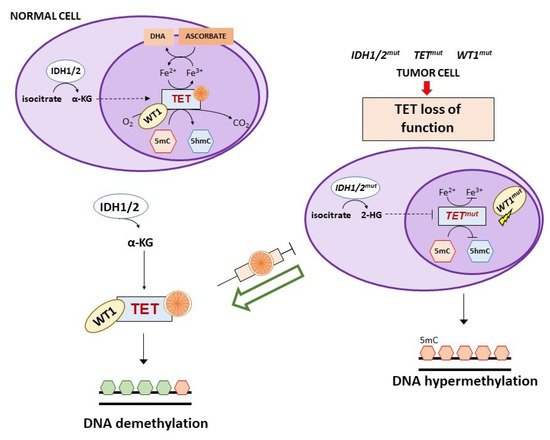
TET2 mutations are mutually exclusive to isocitrate dehydrogenase (IDH) 1/2 or Wilms tumor protein 1 (WT1) mutations that can also be detected in AML and myelodysplastic syndromes [100]. IDH1/2 enzymes catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (αKG) that is required for the activity of multiple dioxygenases, including TETs. Gain-of-function mutations of IDH1/2 result in the overproduction of the oncometabolite 2-hydroxyglutarate (2-HG) that is able to inhibit TET2 through a competitive mechanism (Figure 4) [101][102][103]. In IDH1 mutant mouse bone marrow cells, vitamin C (added daily at 100 μg/mL corresponding to 0.325 mM, in the form of 2-phosphate L-ascorbic acid) was found to overcome the effects of IDH1 mutations, promoting DNA demethylation and epigenetic remodeling of transcription factor-binding sites through stimulation of TET2 activity with consequent induction of leukemia cell differentiation [104]. Of interest, 2-phosphate L-ascorbic acid is a compound that is stable in cell culture and does not induce the production of extracellular H2O2, allowing to study only the activity of vitamin C as enzymatic regulator [104][105]. IDH1/2 mutations are also detected in solid tumors (e.g., glioma, colorectal, breast, renal cancers) and in an IDH1 mutated colorectal cancer cell line, treatment with vitamin C synergized with an IHD1 inhibitor by rescuing TET activity [106].
Prolonged exposure to vitamin C, at concentrations capable of regulating αKGDDs enzymatic activity, may induce epigenomic remodeling. In blast cells of leukemia patients with TET2 mutations, aberrant promoter methylation and reduction in 5hmC at the level of gene enhancers were detected [107]. In human kidney cancer cells lines, restoration of DNA 5hmC levels after protracted vitamin C exposure was also observed [108]. Furthermore, in IDH1 mutant mouse bone marrow cells, vitamin C induced differentiation and maturation of myeloid progenitor cells [104]. Through this mechanism, vitamin C may counteract the epigenetic dysregulation associated with cancer development and progression, which leads to aberrant gene expression and genomic instability.
Pharmacological doses of vitamin C were reported to reduce DNA methylation and to restore 5hmC DNA levels via TET2 activity even in tumors with functional loss of TET2 unrelated to gene mutations or transcriptional inactivation. Recently, low levels of 5hmC have been proposed as an independent adverse prognostic marker in tumors such as cutaneous T-cell lymphoma and clear cell renal cell carcinoma [109][110][111]. The latter tumor shows DNA cytosine hypermethylation, especially at the level of tumor-suppressor genes, that has been attributed to low expression of L-2-hydroxyglutarate dehydrogenase (L2HGDH) with consequent overproduction of the 2HG oncometabolite (the L isoform) that in turn causes functional inactivation of TET2. Treatment with vitamin C reduced DNA methylation and restored 5hmC levels via TET activation and inhibited tumor growth in vitro and in vivo [111].
WT1 is a transcription factor that regulates many cellular pathways, including WNT and MAPK signaling, and is involved in processes like cell differentiation and tumor suppression. This transcription factor interacts with TET2 and recruits it to the promoter of genes regulated by WT1 favoring their demethylation and expression [100][112]. Mutations of WT1 hamper the ability of TET2 to bind to and induce transcriptional activation of WT1-target genes (Figure 4). A clinical study on WT1-mutated AML refractory to induction chemotherapy suggested the use of vitamin C as adjunct therapy, based on the evidence that WT1 mutant leukemia cells show low 5hmC levels that in turn indicate reduced TET2 activity [15]. Since WT1 mutations are present in a large number of tumors, it is likely that vitamin C treatment might be useful also in other clinical settings besides AML [100].
5.3. Vitamin C as Enzymatic Regulator of Others αKGDDs
HIF hydroxylases are another class of αKGDDs enzymes that are affected by vitamin C. In human melanoma cell lines, vitamin C was found to act as cofactor of HIF hydroxylases, which induces recognition of HIF-1α by the von Hippel–Lindau tumor suppressor protein (VHL) with consequent ubiquitination and proteasomal degradation [113]. The heterodimeric transcription factor HIF1 comprises a cytosolic, O2 sensitive subunit (HIF-1α) and a constitutively expressed subunit (HIF-1β). Under oxygen deficiency conditions (e.g., ischemia, tumors), HIF-1α undergoes hydroxylation on specific proline and asparagine residues and proteasomal degradation is prevented. In this way, HIF-1α can translocate to the nucleus, dimerize with HIF-1β and activate target genes involved in the regulation of many cellular functions such as proliferation, apoptosis, cell migration, angiogenesis, glucose transport, and metabolism [114]. In various tumors, HIF-1α is constitutively activated and high levels of expression seem to correlate with vitamin C cytotoxicity [115][116][117]. Moreover, loss of function mutations in succinate dehydrogenase and fumarate dehydrogenase can increase succinate and fumarate levels, which, in turn, may lead to competitive inhibition of HIF hydroxylases and HIF-1α constitutive activation [18][115]. In several human cancers (endometrial, colorectal, breast, and thyroid cancer), an inverse correlation between HIF-1α and intracellular vitamin C levels was also found [118][119][120]. In AML patients, high expression levels of HIF-1α and GLUT1 were associated with lack of response to chemotherapy, probably due to the higher glycolytic metabolism of resistant tumor cells [121]. Moreover, high HIF-1α activity has been reported to inhibit TET2 expression [122]. Therefore, in tumors with HIF1 overexpression or overactivation, vitamin C treatment may increase the activity of HIF hydroxylases, with consequent HIF1α degradation and inhibition of the tumor promoting effects of this transcription factor [18][123][124][125][126]. However, in patients with clear-cell renal cell carcinomas with mutations in the VHL gene that prevent the degradation of hydroxylated HIF-α with consequent accumulation of the transcription factor, there was no association between HIF activity and ascorbate content [127]. These data suggest that vitamin C treatment is unlikely to be effective in VHL-defective tumors. Conversely, in VHL-proficient tumors with increased HIF activity due to hypoxic conditions, high-dose vitamin C might prove beneficial by enhancing HIF-α degradation through stimulation of HIF hydroxylase enzyme activity [127]. Nevertheless, since HIF positively regulates GLUT1 expression, this might favor the entry of DHA into VHL-defective cells sensitizing them to vitamin C cytotoxic effects [115][128]. Interestingly, administration of ascorbate by intravenous infusion to colon cancer patients resulted in increased ascorbate content within the tumor and reduced expression of HIF-dependent proteins [129].
Other epigenetic regulators belonging to the αKGDDs class are the Jumonji C-domain-containing histone demethylases (JHDM). These enzymes catalyze the histone demethylation at arginine and lysine residues regulating chromatin-dependent processes. Some evidence demonstrated that vitamin C may modulate JHDM activity affecting their role in embryonic stem cell reprogramming [130]. However, further studies are required to clarify the ability of vitamin C to modulate JHDM activity in cancer [40].
5.4. Vitamin C may Favor DNA Damage by Increasing 5-Hydroxymethylcytosine Levels
DNA damage induced by vitamin C can occur at in vitro pro-oxidant concentrations (>1 mM), as well as at concentrations capable of regulating αKGDDs enzymatic activity (0.25–1 mM) [98][104][108][131][132]. In the latter case, vitamin C-induced DNA damage derives from its ability to increase 5hmC levels.
In recent years, several studies have demonstrated a fundamental role of 5hmC as the most stable oxidized 5mC intermediate in the DNA demethylation process [133]. As described above, low 5hmC levels were reported as marker of TET dysregulation and vitamin C was found to enhance TET activity mimicking the action of hypomethylating agents [90][98][110][131][134][135][136][137][138][139][140][141][142][143]. In tumor tissues, lower 5hmC levels are generally detected compared to normal tissues that have been attributed to down-regulation of TET activity by tumor hypoxic conditions since O2 is required for TET function [144][145]. Other pathways that can explain the low amount of 5hmC in tumor DNA include: (a) passive dilution by cell division in the presence of defective activity of DNA methyltransferase 1 (DNMT1); (b) changes in TET activity as a consequence of gene mutations (see Section 6.2) or TET protein “de-localization” [146][147].
Vitamin C is known to induce single strand breaks and to activate the BER pathway via TET-mediated DNA oxidation [98][148]. Moreover, the presence of 5hmC at stalled replication forks acts as a recruitment marker for the BER component apurinic and apyrimidinic endonuclease 1 (APE1) [149]. In a BRCA2-deficient murine cellular model, Kharat and colleagues demonstrated that low levels of 5hmC and TET2 expression were associated with increased stability of stalled replication forks and resistance to PARP inhibitors [149]. PARP inhibitors are a class of anticancer drugs approved for the treatment of tumors with defective DNA repair due to germline and somatic mutations or epigenetic alterations of essential components (e.g., BRCA1 or BRCA2) of the homologous recombination system that is involved in the repair of DNA double strand breaks [150]. PARP inhibitor-induced trapping of PARP1 at DNA damage site impairs the progression of the replication fork and the repair/restarting of stalled replication fork requires a fully active homologous recombination. In homologous recombination-deficient cells, the intervention of error-prone DNA repair processes (e.g., non-homologous end-joining) can induce genomic instability and rearrangements, which eventually lead to tumor cell death and synthetic lethality [151][152]. Moreover, PARP1 trapping triggers excessive fork degradation of stalled replication forks resulting in fork collapse and DNA double strand breaks [153]. On this basis, in BRCA-deficient tumors, protection of stalled replication forks may contribute to the development of resistance to PARP inhibitors. In the presence of TET2 functional defects and consequent low 5hmC levels, the recruitment of APE1 on the stalled replication fork is impaired and this impedes the degradation of stalled replication forks [149]. In this context, exposure to vitamin C was found to increase 5hmC via TET2 activity, by restoring the recruitment of APE1 on stalled replication forks and inducing their degradation [149][154]. However, additional studies are required to establish whether vitamin C, by increasing 5hmC levels, might contribute to restore the sensitivity of tumors resistant to PARPi or to other chemotherapeutic agents that induce a DNA damage response involving the homologous recombination system (e.g., cisplatin).
This entry is adapted from the peer-reviewed paper 10.3390/cancers13061428
References
- Carpenter, K.J. The Discovery of Vitamin C. Ann. Nutr. Metab. 2012, 61, 259–264.
- Svirbely, J.L.; Szent-Györgyi, A. The chemical nature of vitamin C. Biochem. J. 1932, 26, 865–870.
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54, 1135S–1140S.
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709.
- Levine, M.; Rumsey, S.C.; Daruwala, R.; Park, J.B.; Wang, Y. Criteria and recommendations for vitamin C intake. J. Am. Med. Assoc. 1999.
- Weinstein, M.; Babyn, P.; Zlotkin, S. An Orange a Day Keeps the Doctor Away: Scurvy in the Year 2000. Pediatrics 2001, 108, e55.
- Wang, A.H.; Still, C. Old World Meets Modern: A Case Report of Scurvy. Nutr. Clin. Pract. 2007, 22, 445–448.
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235.
- Cameron, E.; Pauling, L. The orthomolecular treatment of cancer I. The role of ascorbic acid in host resistance. Chem. Biol. Interact. 1974, 9, 273–283.
- Cameron, E.; Campbell, A. The orthomolecular treatment of cancer II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 1974.
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976.
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of High-Dose Vitamin C (Ascorbic Acid) Therapy to Benefit Patients with Advanced Cancer. N. Engl. J. Med. 1979.
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-Dose Vitamin C versus Placebo in the Treatment of Patients with Advanced Cancer Who Have Had No Prior Chemotherapy. N. Engl. J. Med. 1985.
- Pauling, L.; Moertel, C. A Proposition: Megadoses of Vitamin C are Valuable in the Treatment of Cancer. Nutr. Rev. 1986, 44, 28–29.
- Das, A.B.; Kakadia, P.M.; Wojcik, D.; Pemberton, L.; Browett, P.J.; Bohlander, S.K.; Vissers, M.C.M. Clinical remission following ascorbate treatment in a case of acute myeloid leukemia with mutations in TET2 and WT1. Blood Cancer J. 2019, 9, 82.
- Bielski, B.H.J.; Allen, A.O.; Schwarz, H.A. Mechanism of the disproportionation of ascorbate radicals. J. Am. Chem. Soc. 1981, 103, 3516–3518.
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta-Rev. Cancer 2012, 1826, 443–457.
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282.
- Astuya, A.; Caprile, T.; Castro, M.; Salazar, K.; García, M.d.l.A.; Reinicke, K.; Rodríguez, F.; Vera, J.C.; Millán, C.; Ulloa, V.; et al. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J. Neurosci. Res. 2005, 79, 146–156.
- Padayatty, S.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493.
- Myllylä, R.; Kuutti-Savolainen, E.-R.; Kivirikko, K.I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem. Biophys. Res. Commun. 1978, 83, 441–448.
- May, J.M.; Qu, Z.; Meredith, M.E. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152.
- May, J.M.; Qu, Z.; Nazarewicz, R.; Dikalov, S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res. Bull. 2013, 90, 35–42.
- Frei, B. Ascorbic acid protects lipids in human plasma and low-density lipoprotein against oxidative damage. Am. J. Clin. Nutr. 1991, 54, 1113S–1118S.
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, α-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543.
- Przybyło, M.; Langner, M. On the physiological and cellular homeostasis of ascorbate. Cell. Mol. Biol. Lett. 2020, 25, 32.
- May, J.M. The SLC23 family of ascorbate transporters: Ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 2011, 164, 1793–1801.
- Godoy, A.; Ormazabal, V.; Moraga-Cid, G.; Zúñiga, F.A.; Sotomayor, P.; Barra, V.; Vasquez, O.; Montecinos, V.; Mardones, L.; Guzmán, C.; et al. Mechanistic Insights and Functional Determinants of the Transport Cycle of the Ascorbic Acid Transporter SVCT2. J. Biol. Chem. 2007, 282, 615–624.
- May, J.M.; Qu, Z.; Qiao, H. Transfer of ascorbic acid across the vascular endothelium: Mechanism and self-regulation. Am. J. Physiol. Physiol. 2009, 297, C169–C178.
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.-Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75.
- Boyer, J.C.; Campbell, C.E.; Sigurdson, W.J.; Kuo, S.-M. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem. Biophys. Res. Commun. 2005, 334, 150–156.
- Jin, S.N.; Mun, G.H.; Lee, J.H.; Oh, C.S.; Kim, J.; Chung, Y.H.; Kang, J.S.; Kim, J.-G.; Hwang, D.-H.; Hwang, Y.I.; et al. Immunohistochemical study on the distribution of sodium-dependent vitamin C transporters in the respiratory system of adult rat. Microsc. Res. Tech. 2005, 68, 360–367.
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Investig. 2010, 120, 1069–1083.
- Sotiriou, S.; Gispert, S.; Cheng, J.; Wang, Y.; Chen, A.; Hoogstraten-Miller, S.; Miller, G.F.; Kwon, O.; Levine, M.; Guttentag, S.H.; et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 2002, 8, 514–517.
- May, J.M.; Qu, Z.; Qiao, H.; Koury, M.J. Maturational loss of the vitamin C transporter in erythrocytes. Biochem. Biophys. Res. Commun. 2007, 360, 295–298.
- Ferrada, L.; Salazar, K.; Nualart, F. Metabolic control by dehydroascorbic acid: Questions and controversies in cancer cells. J. Cell. Physiol. 2019, 234, 19331–19338.
- Wilson, J.X. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005, 25, 105–125.
- Savini, I.; Catani, M.V.; Arnone, R.; Rossi, A.; Frega, G.; Del Principe, D.; Avigliano, L. Translational control of the ascorbic acid transporter SVCT2 in human platelets. Free Radic. Biol. Med. 2007, 42, 608–616.
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal Dehydroascorbic Acid (DHA) Transport Mediated by the Facilitative Sugar Transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101.
- Gillberg, L.; Ørskov, A.D.; Liu, M.; Harsløf, L.B.S.; Jones, P.A.; Grønbæk, K. Vitamin C—A new player in regulation of the cancer epigenome. Semin. Cancer Biol. 2018, 51, 59–67.
- Wang, Y.; Mackenzie, B.; Tsukaguchi, H.; Weremowicz, S.; Morton, C.C.; Hediger, M.A. Human Vitamin C (l-Ascorbic Acid) Transporter SVCT1. Biochem. Biophys. Res. Commun. 2000, 267, 488–494.
- Malo, C.; Wilson, J.X. Glucose Modulates Vitamin C Transport in Adult Human Small Intestinal Brush Border Membrane Vesicles. J. Nutr. 2000, 130, 63–69.
- Vera, J.C.; Rivas, C.I.; Fischbarg, J.; Golde, D.W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993, 364, 79–82.
- Vera, J.; Rivas, C.; Zhang, R.; Farber, C.; Golde, D. Human HL-60 myeloid leukemia cells transport dehydroascorbic acid via the glucose transporters and accumulate reduced ascorbic acid. Blood 1994, 84, 1628–1634.
- Vera, J.C.; Rivas, C.I.; Velásquez, F.V.; Zhang, R.H.; Concha, I.I.; Golde, D.W. Resolution of the Facilitated Transport of Dehydroascorbic Acid from Its Intracellular Accumulation as Ascorbic Acid. J. Biol. Chem. 1995, 270, 23706–23712.
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396.
- Lu, Y.-X.; Wu, Q.-N.; Chen, D.; Chen, L.-Z.; Wang, Z.-X.; Ren, C.; Mo, H.; Chen, Y.; Sheng, H.; Wang, Y.-N.; et al. Pharmacological Ascorbate Suppresses Growth of Gastric Cancer Cells with GLUT1 Overexpression and Enhances the Efficacy of Oxaliplatin Through Redox Modulation. Theranostics 2018, 8, 1312–1326.
- Kallner, A.; Hartmann, D.; Hornig, D. Steady-state turnover and body pool of ascorbic acid in man. Am. J. Clin. Nutr. 1979, 32, 530–539.
- Graumlich, J.F.; Ludden, T.M.; Conry-Cantilena, C.; Cantilena, L.R.; Wang, Y.; Levine, M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm. Res. 1997, 14, 1133–1139.
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Poulsen, H.E.; Lykkesfeldt, J.; Mikines, K.J. Elimination of Ascorbic Acid After High-Dose Infusion in Prostate Cancer Patients: A Pharmacokinetic Evaluation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 343–348.
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412.
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846.
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533.
- Berger, L.; Gerson, C.D.; Yü, T.-F. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am. J. Med. 1977, 62, 71–76.
- Hickey, D.S.; Roberts, H.J.; Cathcart, R.F. Dynamic flow: A new model for ascorbate. J. Orthomol. Med. 2005, 20, 237–244.
- Duconge, J.; Miranda-Massari, J.R.; Gonzalez, M.J.; Jackson, J.A.; Warnock, W.; Riordan, N.H. Pharmacokinetics of vitamin C: Insights into the oral and intravenous administration of ascorbate. Puerto Rico Health Sci. J. 2008, 27, 7–19.
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013.
- Mahdavi, R.; Faramarzi, E.; Seyedrezazadeh, E.; Mohammad-zadeh, M.; Pourmoghaddam, M. Evaluation of Oxidative Stress, Antioxidant Status and Serum Vitamin C Levels in Cancer Patients. Biol. Trace Elem. Res. 2009, 130, 1–6.
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10, 189.
- Mikirova, N.; Casciari, J.; Riordan, N.; Hunninghake, R. Clinical experience with intravenous administration of ascorbic acid: Achievable levels in blood for different states of inflammation and disease in cancer patients. J. Transl. Med. 2013, 11, 191.
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609.
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.-H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754.
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109.
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of Ascorbate-Induced Cytotoxicity in Pancreatic Cancer. Clin. Cancer Res. 2010, 16, 509–520.
- Takemura, Y.; Satoh, M.; Satoh, K.; Hamada, H.; Sekido, Y.; Kubota, S. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem. Biophys. Res. Commun. 2010, 394, 249–253.
- Pollard, H.B.; Levine, M.A.; Eidelman, O.; Pollard, M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo 2010, 24, 249–255.
- Chen, P.; Yu, J.; Chalmers, B.; Drisko, J.; Yang, J.; Li, B.; Chen, Q. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs 2012, 23, 437–444.
- Venturelli, S.; Sinnberg, T.W.; Berger, A.; Noor, S.; Levesque, M.P.; Böcker, A.; Niessner, H.; Lauer, U.M.; Bitzer, M.; Garbe, C.; et al. Epigenetic Impacts of Ascorbate on Human Metastatic Melanoma Cells. Front. Oncol. 2014, 4.
- Ma, E.; Chen, P.; Wilkins, H.M.; Wang, T.; Swerdlow, R.H.; Chen, Q. Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic. Biol. Med. 2017, 113, 36–47.
- O’Leary, B.R.; Alexander, M.S.; Du, J.; Moose, D.L.; Henry, M.D.; Cullen, J.J. Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci. Rep. 2020, 10, 17649.
- McCarty, M.F.; Contreras, F. Increasing Superoxide Production and the Labile Iron Pool in Tumor Cells may Sensitize Them to Extracellular Ascorbate. Front. Oncol. 2014, 4, 1–9.
- Rivière, J.; Ravanat, J.-L.; Wagner, J.R. Ascorbate and H2O2 induced oxidative DNA damage in Jurkat cells. Free Radic. Biol. Med. 2006, 40, 2071–2079.
- Sinnberg, T.; Noor, S.; Venturelli, S.; Berger, A.; Schuler, P.; Garbe, C.; Busch, C. The ROS-induced cytotoxicity of ascorbate is attenuated by hypoxia and HIF-1 alpha in the 60 cancer cell lines. J. Cell. Mol. Med. 2014, 18, 530–541.
- Gibson, A.R.; O’Leary, B.R.; Du, J.; Sarsour, E.H.; Kalen, A.L.; Wagner, B.A.; Stolwijk, J.M.; Falls-Hubert, K.C.; Alexander, M.S.; Carroll, R.S.; et al. Dual Oxidase-Induced Sustained Generation of Hydrogen Peroxide Contributes to Pharmacologic Ascorbate-Induced Cytotoxicity. Cancer Res. 2020, 80, 1401–1413.
- Buranasudja, V.; Doskey, C.M.; Gibson, A.R.; Wagner, B.A.; Du, J.; Gordon, D.J.; Koppenhafer, S.L.; Cullen, J.J.; Buettner, G.R. Pharmacologic Ascorbate Primes Pancreatic Cancer Cells for Death by Rewiring Cellular Energetics and Inducing DNA Damage. Mol. Cancer Res. 2019, 17, 2102–2114.
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 13896.
- Ghanem, A.; Melzer, A.M.; Zaal, E.; Neises, L.; Baltissen, D.; Matar, O.; Glennemeier-Marke, H.; Almouhanna, F.; Theobald, J.; Abu el Maaty, M.A.; et al. Ascorbate kills breast cancer cells by rewiring metabolism via redox imbalance and energy crisis. Free Radic. Biol. Med. 2021, 163, 196–209.
- Schraufstätter, I.U.; Hinshaw, D.B.; Hyslop, P.A.; Spragg, R.G.; Cochrane, C.G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J. Clin. Investig. 1985, 76, 1131–1139.
- Lee, Y.; Shacter, E. Oxidative Stress Inhibits Apoptosis in Human Lymphoma Cells. J. Biol. Chem. 1999, 274, 19792–19798.
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383.
- Choi, J.-A.; Lee, J.-W.; Kim, H.; Kim, E.-Y.; Seo, J.-M.; Ko, J.; Kim, J.-H. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2–reactive oxygen species-linked signaling pathway. Carcinogenesis 2010, 31, 543–551.
- Luanpitpong, S.; Talbott, S.J.; Rojanasakul, Y.; Nimmannit, U.; Pongrakhananon, V.; Wang, L.; Chanvorachote, P. Regulation of Lung Cancer Cell Migration and Invasion by Reactive Oxygen Species and Caveolin-1. J. Biol. Chem. 2010, 285, 38832–38840.
- Cichon, M.A.; Radisky, D.C. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-κB-dependent activation of Snail. Oncotarget 2014, 5, 2827–2838.
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483.
- Wei, X.; Xu, Y.; Xu, F.F.; Chaiswing, L.; Schnell, D.; Noel, T.; Wang, C.; Chen, J.; St. Clair, D.K.; St. Clair, W.H. RelB Expression Determines the Differential Effects of Ascorbic Acid in Normal and Cancer Cells. Cancer Res. 2017, 77, 1345–1356.
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691.
- Noguera, N.I.; Pelosi, E.; Angelini, D.F.; Piredda, M.L.; Guerrera, G.; Piras, E.; Battistini, L.; Massai, L.; Berardi, A.; Catalano, G.; et al. High-dose ascorbate and arsenic trioxide selectively kill acute myeloid leukemia and acute promyelocytic leukemia blasts in vitro. Oncotarget 2017, 8, 32550–32565.
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients 2019, 11, 1926.
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate Induces Ten-Eleven Translocation (Tet) Methylcytosine Dioxygenase-mediated Generation of 5-Hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674.
- Yin, R.; Mao, S.-Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic Acid Enhances Tet-Mediated 5-Methylcytosine Oxidation and Promotes DNA Demethylation in Mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403.
- Dickson, K.M.; Gustafson, C.B.; Young, J.I.; Züchner, S.; Wang, G. Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate. Biochem. Biophys. Res. Commun. 2013, 439, 522–527.
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226.
- Chen, J.; Guo, L.; Zhang, L.; Wu, H.; Yang, J.; Liu, H.; Wang, X.; Hu, X.; Gu, T.; Zhou, Z.; et al. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat. Genet. 2013, 45, 1504–1509.
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481.
- Delhommeau, F.; Dupont, S.; Valle, V.D.; James, C.; Trannoy, S.; Massé, A.; Kosmider, O.; Le Couedic, J.-P.; Robert, F.; Alberdi, A.; et al. Mutation in TET2 in Myeloid Cancers. N. Engl. J. Med. 2009, 360, 2289–2301.
- Quivoron, C.; Couronné, L.; Della Valle, V.; Lopez, C.K.; Plo, I.; Wagner-Ballon, O.; Do Cruzeiro, M.; Delhommeau, F.; Arnulf, B.; Stern, M.-H.; et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer Cell 2011, 20, 25–38.
- Tefferi, A.; Pardanani, A.; Lim, K.-H.; Abdel-Wahab, O.; Lasho, T.L.; Patel, J.; Gangat, N.; Finke, C.M.; Schwager, S.; Mullally, A.; et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia 2009, 23, 905–911.
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095.e20.
- Guan, Y.; Greenberg, E.F.; Hasipek, M.; Chen, S.; Liu, X.; Kerr, C.M.; Gackowski, D.; Zarakowska, E.; Radivoyevitch, T.; Gu, X.; et al. Context dependent effects of ascorbic acid treatment in TET2 mutant myeloid neoplasia. Commun. Biol. 2020, 3, 493.
- Wang, Y.; Xiao, M.; Chen, X.; Chen, L.; Xu, Y.; Lv, L.; Wang, P.; Yang, H.; Ma, S.; Lin, H.; et al. WT1 Recruits TET2 to Regulate Its Target Gene Expression and Suppress Leukemia Cell Proliferation. Mol. Cell 2015, 57, 662–673.
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567.
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T.; et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 2011, 19, 17–30.
- Gagné, L.M.; Boulay, K.; Topisirovic, I.; Huot, M.-É.; Mallette, F.A. Oncogenic Activities of IDH1/2 Mutations: From Epigenetics to Cellular Signaling. Trends Cell Biol. 2017, 27, 738–752.
- Mingay, M.; Chaturvedi, A.; Bilenky, M.; Cao, Q.; Jackson, L.; Hui, T.; Moksa, M.; Heravi-Moussavi, A.; Humphries, R.K.; Heuser, M.; et al. Vitamin C-induced epigenomic remodelling in IDH1 mutant acute myeloid leukaemia. Leukemia 2018, 32, 11–20.
- Takamizawa, S. Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol. Int. 2004, 28, 255–265.
- Gerecke, C.; Schumacher, F.; Berndzen, A.; Homann, T.; Kleuser, B. Vitamin C in combination with inhibition of mutant IDH1 synergistically activates TET enzymes and epigenetically modulates gene silencing in colon cancer cells. Epigenetics 2020, 15, 307–322.
- Rasmussen, K.D.; Jia, G.; Johansen, J.V.; Pedersen, M.T.; Rapin, N.; Bagger, F.O.; Porse, B.T.; Bernard, O.A.; Christensen, J.; Helin, K. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Genes Dev. 2015, 29, 910–922.
- Ge, G.; Peng, D.; Xu, Z.; Guan, B.; Xin, Z.; He, Q.; Zhou, Y.; Li, X.; Zhou, L.; Ci, W. Restoration of 5-hydroxymethylcytosine by ascorbate blocks kidney tumour growth. EMBO Rep. 2018, 19.
- Qiu, L.; Liu, F.; Yi, S.; Li, X.; Liu, X.; Xiao, C.; Lian, C.G.; Tu, P.; Wang, Y. Loss of 5-Hydroxymethylcytosine Is an Epigenetic Biomarker in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 2388–2397.
- Peng, D.; Ge, G.; Gong, Y.; Zhan, Y.; He, S.; Guan, B.; Li, Y.; Xu, Z.; Hao, H.; He, Z.; et al. Vitamin C increases 5-hydroxymethylcytosine level and inhibits the growth of bladder cancer. Clin. Epigenet. 2018, 10, 94.
- Shenoy, N.; Bhagat, T.D.; Cheville, J.; Lohse, C.; Bhattacharyya, S.; Tischer, A.; Machha, V.; Gordon-Mitchell, S.; Choudhary, G.; Wong, L.-F.; et al. Ascorbic acid–induced TET activation mitigates adverse hydroxymethylcytosine loss in renal cell carcinoma. J. Clin. Investig. 2019, 129, 1612–1625.
- Rampal, R.; Alkalin, A.; Madzo, J.; Vasanthakumar, A.; Pronier, E.; Patel, J.; Li, Y.; Ahn, J.; Abdel-Wahab, O.; Shih, A.; et al. DNA Hydroxymethylation Profiling Reveals that WT1 Mutations Result in Loss of TET2 Function in Acute Myeloid Leukemia. Cell Rep. 2014, 9, 1841–1855.
- Fischer, A.P.; Miles, S.L. Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease Hypoxia Inducible Factor -1 alpha activity and reduce malignant potential in human melanoma. Biomed. Pharmacother. 2017, 86, 502–513.
- Kuiper, C.; Vissers, M.C.M. Ascorbate as a Co-Factor for Fe- and 2-Oxoglutarate Dependent Dioxygenases: Physiological Activity in Tumor Growth and Progression. Front. Oncol. 2014, 4.
- Kuiper, C.; Molenaar, I.G.M.; Dachs, G.U.; Currie, M.J.; Sykes, P.H.; Vissers, M.C.M. Low Ascorbate Levels Are Associated with Increased Hypoxia-Inducible Factor-1 Activity and an Aggressive Tumor Phenotype in Endometrial Cancer. Cancer Res. 2010, 70, 5749–5758.
- Campbell, E.J.; Vissers, M.C.M.; Wohlrab, C.; Hicks, K.O.; Strother, R.M.; Bozonet, S.M.; Robinson, B.A.; Dachs, G.U. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic. Biol. Med. 2016, 99, 451–462.
- Wohlrab, C.; Kuiper, C.; Vissers, M.C.; Phillips, E.; Robinson, B.A.; Dachs, G.U. Ascorbate modulates the hypoxic pathway by increasing intracellular activity of the HIF hydroxylases in renal cell carcinoma cells. Hypoxia 2019, 7, 17–31.
- Kuiper, C.; Dachs, G.U.; Munn, D.; Currie, M.J.; Robinson, B.A.; Pearson, J.F.; Vissers, M.C.M. Increased Tumor Ascorbate is Associated with Extended Disease-Free Survival and Decreased Hypoxia-Inducible Factor-1 Activation in Human Colorectal Cancer. Front. Oncol. 2014, 4.
- Campbell, E.J.; Dachs, G.U.; Morrin, H.R.; Davey, V.C.; Robinson, B.A.; Vissers, M.C.M. Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels. BMC Cancer 2019, 19, 307.
- Jóźwiak, P.; Ciesielski, P.; Zaczek, A.; Lipińska, A.; Pomorski, L.; Wieczorek, M.; Bryś, M.; Forma, E.; Krześlak, A. Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions. J. Biomed. Sci. 2017, 24, 83.
- Song, K.; Li, M.; Xu, X.-J.; Xuan, L.; Huang, G.-N.; Song, X.-L.; Liu, Q.-F. HIF-1α and GLUT1 Gene Expression is Associated with Chemoresistance of Acute Myeloid Leukemia. Asian Pac. J. Cancer Prev. 2014, 15, 1823–1829.
- Fischer, A.P.; Miles, S.L. Silencing HIF-1α induces TET2 expression and augments ascorbic acid induced 5-hydroxymethylation of DNA in human metastatic melanoma cells. Biochem. Biophys. Res. Commun. 2017, 490, 176–181.
- Knowles, H.J.; Raval, R.R.; Harris, A.L.; Ratcliffe, P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003, 63, 1764–1768.
- Kawada, H.; Kaneko, M.; Sawanobori, M.; Uno, T.; Matsuzawa, H.; Nakamura, Y.; Matsushita, H.; Ando, K. High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1α transcription. PLoS ONE 2013, 8, e62717.
- Miles, S.L.; Fischer, A.P.; Joshi, S.J.; Niles, R.M. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer 2015, 15, 867.
- Wilkes, J.G.; O’Leary, B.R.; Du, J.; Klinger, A.R.; Sibenaller, Z.A.; Doskey, C.M.; Gibson-Corley, K.N.; Alexander, M.S.; Tsai, S.; Buettner, G.R.; et al. Pharmacologic ascorbate (P-AscH−) suppresses hypoxia-inducible Factor-1α (HIF-1α) in pancreatic adenocarcinoma. Clin. Exp. Metastasis 2018, 35, 37–51.
- Wohlrab, C.; Vissers, M.C.M.; Phillips, E.; Morrin, H.; Robinson, B.A.; Dachs, G.U. The Association Between Ascorbate and the Hypoxia-Inducible Factors in Human Renal Cell Carcinoma Requires a Functional Von Hippel-Lindau Protein. Front. Oncol. 2018, 8.
- Tian, W.; Wang, Y.; Xu, Y.; Guo, X.; Wang, B.; Sun, L.; Liu, L.; Cui, F.; Zhuang, Q.; Bao, X.; et al. The Hypoxia-inducible Factor Renders Cancer Cells More Sensitive to Vitamin C-induced Toxicity. J. Biol. Chem. 2014, 289, 3339–3351.
- Dachs, G.U.; Gandhi, J.; Wohlrab, C.; Carr, A.C.; Morrin, H.R.; Pullar, J.M.; Bayer, S.B.; Eglinton, T.W.; Robinson, B.A.; Vissers, M.C.M. Vitamin C Administration by Intravenous Infusion Increases Tumor Ascorbate Content in Patients With Colon Cancer: A Clinical Intervention Study. Front. Oncol. 2021, 10.
- Ebata, K.T.; Mesh, K.; Liu, S.; Bilenky, M.; Fekete, A.; Acker, M.G.; Hirst, M.; Garcia, B.A.; Ramalho-Santos, M. Vitamin C induces specific demethylation of H3K9me2 in mouse embryonic stem cells via Kdm3a/b. Epigenet. Chromatin 2017, 10, 36.
- Huang, R.; Wang, Y.; Ge, H.; Wang, D.; Wang, Y.; Zhang, W.; Yang, J.; Cheng, J. Restoration of TET2 deficiency inhibits tumor growth in head neck squamous cell carcinoma. Ann. Transl. Med. 2020, 8, 329.
- Ma, Y.; Chen, P.; Drisko, J.; Khabele, D.; Godwin, A.; Chen, Q. Pharmacological ascorbate induces ‘BRCAness’ and enhances the effects of Poly(ADP-Ribose) polymerase inhibitors against BRCA1/2 wild-type ovarian cancer. Oncol. Lett. 2020, 19.
- Bachman, M.; Uribe-Lewis, S.; Yang, X.; Williams, M.; Murrell, A.; Balasubramanian, S. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat. Chem. 2014, 6, 1049–1055.
- Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol. 2018, 28, 698–708.
- Starczak, M.; Zarakowska, E.; Modrzejewska, M.; Dziaman, T.; Szpila, A.; Linowiecka, K.; Guz, J.; Szpotan, J.; Gawronski, M.; Labejszo, A.; et al. In vivo evidence of ascorbate involvement in the generation of epigenetic DNA modifications in leukocytes from patients with colorectal carcinoma, benign adenoma and inflammatory bowel disease. J. Transl. Med. 2018, 16, 204.
- Sant, D.W.; Mustafi, S.; Gustafson, C.B.; Chen, J.; Slingerland, J.M.; Wang, G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci. Rep. 2018, 8, 5306.
- Siref, A.; McCormack, C.; Huang, Q.; Lim, W.; Alkan, S. Diminished expression of 5hmc in Reed-Sternberg cells in classical Hodgkin lymphoma is a common epigenetic marker. Leuk. Res. 2020, 96, 106408.
- Haffner, M.C.; Chaux, A.; Meeker, A.K.; Esopi, D.M.; Gerber, J.; Pellakuru, L.G.; Toubaji, A.; Argani, P.; Iacobuzio-Donahue, C.; Nelson, W.G.; et al. Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget 2011, 2, 627–637.
- Kudo, Y.; Tateishi, K.; Yamamoto, K.; Yamamoto, S.; Asaoka, Y.; Ijichi, H.; Nagae, G.; Yoshida, H.; Aburatani, H.; Koike, K. Loss of 5-hydroxymethylcytosine is accompanied with malignant cellular transformation. Cancer Sci. 2012, 103, 670–676.
- Lian, C.G.; Xu, Y.; Ceol, C.; Wu, F.; Larson, A.; Dresser, K.; Xu, W.; Tan, L.; Hu, Y.; Zhan, Q.; et al. Loss of 5-Hydroxymethylcytosine Is an Epigenetic Hallmark of Melanoma. Cell 2012, 150, 1135–1146.
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.-J.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenetics 2015, 7, 51.
- Shenoy, N.; Bhagat, T.; Nieves, E.; Stenson, M.; Lawson, J.; Choudhary, G.S.; Habermann, T.; Nowakowski, G.; Singh, R.; Wu, X.; et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017, 7, e587.
- Mustafi, S.; Sant, D.W.; Liu, Z.-J.; Wang, G. Ascorbate induces apoptosis in melanoma cells by suppressing Clusterin expression. Sci. Rep. 2017, 7, 3671.
- Thienpont, B.; Steinbacher, J.; Zhao, H.; D’Anna, F.; Kuchnio, A.; Ploumakis, A.; Ghesquière, B.; Van Dyck, L.; Boeckx, B.; Schoonjans, L.; et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 2016, 537, 63–68.
- Pomerleau, J.; Weidmann, C.; Coutant, K.; Lowry, C.-M.; Veilleux, M.-P.; Bérubé, J.; Wagner, J.R.; Landreville, S. Experimental eye research/short communication format characterization of DNA hydroxymethylation in the ocular choroid. Exp. Eye Res. 2021, 205, 108473.
- Ficz, G.; Gribben, J.G. Loss of 5-hydroxymethylcytosine in cancer: Cause or consequence? Genomics 2014, 104, 352–357.
- Huang, Y.; Wang, G.; Liang, Z.; Yang, Y.; Cui, L.; Liu, C.-Y. Loss of nuclear localization of TET2 in colorectal cancer. Clin. Epigenetics 2016, 8, 9.
- Singh, N.P. Sodium ascorbate induces DNA single-strand breaks in human cells in vitro. Mutat. Res. Mol. Mech. Mutagen. 1997, 375, 195–203.
- Kharat, S.S.; Ding, X.; Swaminathan, D.; Suresh, A.; Singh, M.; Sengodan, S.K.; Burkett, S.; Marks, H.; Pamala, C.; He, Y.; et al. Degradation of 5hmC-marked stalled replication forks by APE1 causes genomic instability. Sci. Signal. 2020, 13, eaba8091.
- Faraoni, I.; Graziani, G. Role of BRCA Mutations in Cancer Treatment with Poly(ADP-ribose) Polymerase (PARP) Inhibitors. Cancers 2018, 10, 487.
- Shah, G.M.; Robu, M.; Purohit, N.K.; Rajawat, J.; Tentori, L.; Graziani, G. PARP Inhibitors in Cancer Therapy: Magic Bullets but Moving Targets. Front. Oncol. 2013, 3.
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158.
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394.
- Ray Chaudhuri, A.; Callen, E.; Ding, X.; Gogola, E.; Duarte, A.A.; Lee, J.-E.; Wong, N.; Lafarga, V.; Calvo, J.A.; Panzarino, N.J.; et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 2016, 535, 382–387.
